This application note demonstrates the transferability of an SEC method on both the ACQUITY Arc Bio System and the Agilent 1260 Infinity Bio-inert System.
In the biopharmaceutical industry, it is common to evaluate biotherapeutics, including monoclonal antibodies (mAb) under their native conditions. This requires the use of aqueous mobile phases containing high salt concentrations. Under these conditions, bio-inert or biocompatible systems are preferred to reduce potential corrosion from the high salt concentration and to avoid oxidation of the protein by the presence of iron ions. There are many biocompatible systems, and each one can consist of different biocompatible materials, such as, MP35N, a nickel-cobalt alloy, titanium, and polyether ether ketone (PEEK) just to name a few.
This study will demonstrate the transfer of a size-exclusion chromatography (SEC) method for a monoclonal antibody biotherapetuic, trastuzumab, across different biocompatible liquid chromatography systems. SEC has been the predominant separation mode for evaluating protein aggregation, including high molecular weight forms (HMW). This area is of particular importance because aggregates and HMW have been shown to correlate with undesired immunogenic effects1 as well as decreased efficacy. Therefore, during SEC method transfer, it is important that the method can quantify the amounts of HMW degradants contained within a sample reproducibly, regardless of the instrumentation used. To illustrate this, we will demonstrate the transferability of an SEC method on both the ACQUITY Arc Bio System and the Agilent 1260 Infinity Bio-inert System. In addition, the impact of diode array and dual wavelength detectors on data repeatability will be studied.
Trastuzumab was obtained at 21 mg/mL. It was analyzed past expiry. The sample was diluted in mobile phase to 2.1 mg/mL.
|
LC system |
ACQUITY Arc Bio System and Agilent 1260 Infinity Bio-inert System |
|
Column: |
XBridge Protein BEH SEC, 200Å, 3.5 μm, 7.8 × 300 mm |
|
Column temp.: |
Ambient |
|
Sample temp.: |
4 °C |
|
Injection volume: |
10 μL |
|
Flow rate: |
0.7 mL/min |
|
Mobile phase: |
25 mM sodium phosphate, 400 mM sodium chloride, pH 6.7 |
|
Run time: |
40 min |


Empower 3 CDS, FR3, and Agilent OpenLab CDS Chemstation
Data was acquired using the system’s native software and processed in Empower.
The Waters Data Converter was used to transfer the OpenLab CDS Chemstation data into Empower.
SEC is a common analytical tool to assess the presence of both HMW and low molecular weight degradants (LMW) forms in the product life cycle of mAbs and other protein-based drugs. Protein aggregates, or HMW, are important degradants of mAb that can potentially impact product safety and efficacy. An additional degradation pathway for mAb is non-enzymatic peptide bond hydrolysis resulting in antibodies missing one or both antigen-binding arms (Fab), which are referred to as LMW.2
An SEC method for trastuzumab, an anti-HER2 IgG1 mAb for treatment of breast cancer,3 was run on the two biocompatible LC systems, an ACQUITY Arc Bio System and an Agilent 1260 Infinity Bio-inert System. Comparable chromatographic profiles with similar retention times and resolution were observed between the two systems (Figure 1). In order to further assess the data, the average retention times and peak areas for three replicate injections were calculated along with the corresponding standard deviations and RSD% (Table 1 and 2). In the both chromatograms, a single HMW and a LMW peak were baseline resolved from the main monomer peak. A non-resolved shoulder (*) was observed adjacent to the monomer peak. For method transferability purpose, the monomer peak was integrated to include this shoulder.
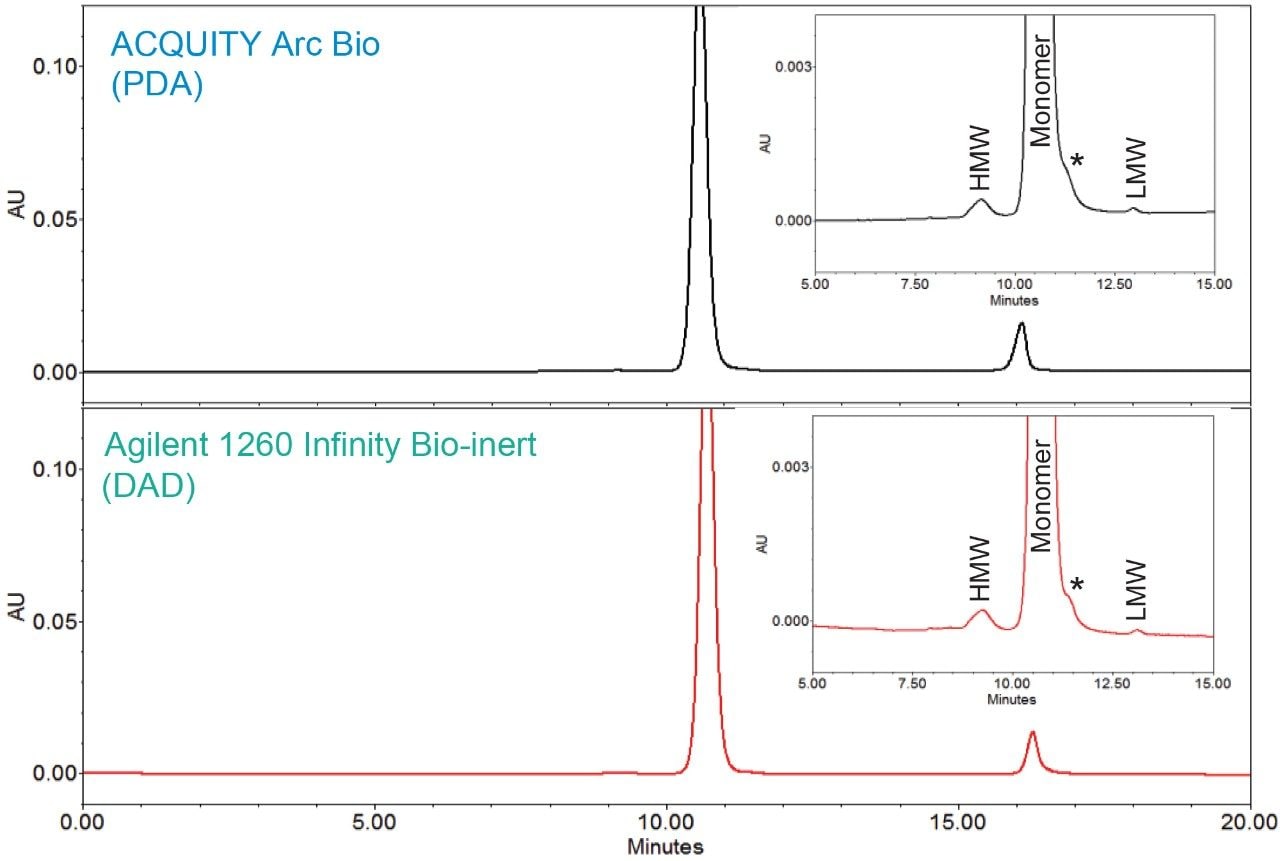
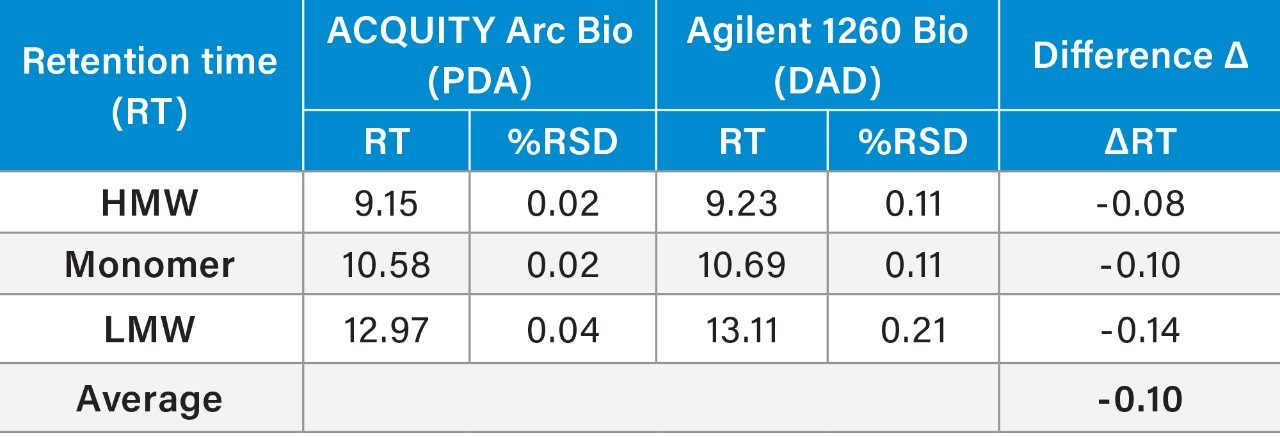
Analyses of the data showed the average retention times (RT) of the three peaks, along with %RSD and the total RT difference between these two systems were comparable. Evaluation of RT also confirmed a high degree similarity between these instruments: the shift in retention time was approximately 0.1 min across the two systems. The RT repeatability for all the peaks showed the %RSD was within 0.2% across both systems. However, the %RSD’s observed on the ACQUITY Arc Bio System were slightly lower than those determined on the Agilent 1260 Infinity Bio-inert System.
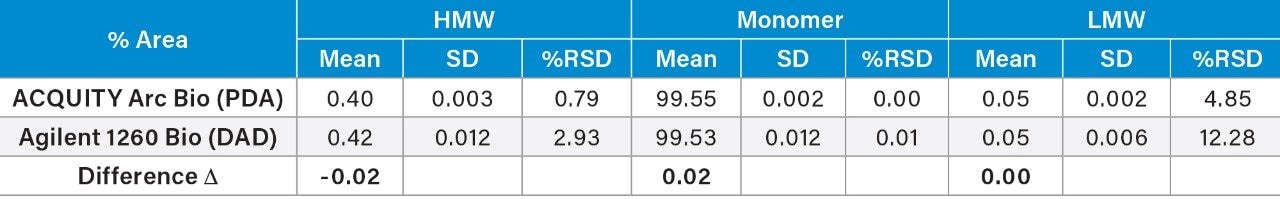
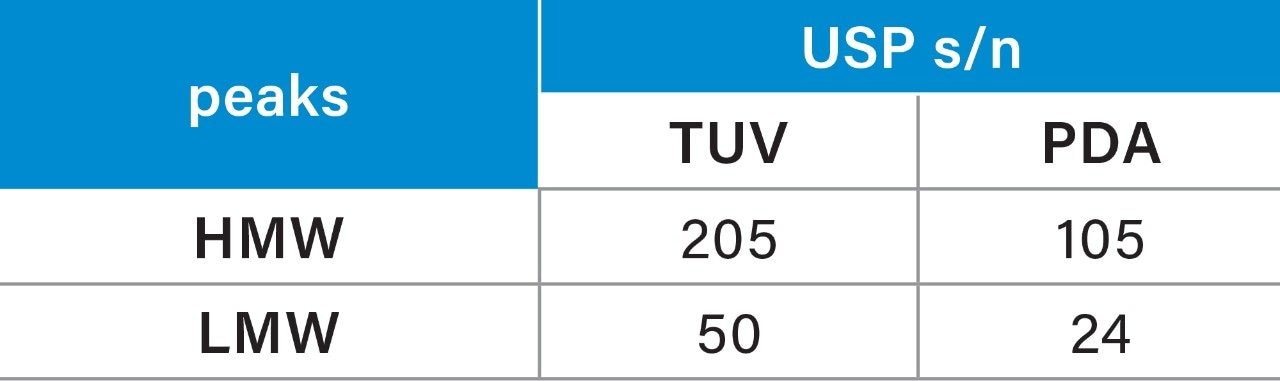
Given the previously described immunogenic response and decreased efficacy, aggregation is a major problem in the development of protein therapeutics. One of the primary goals of the SEC separation is to measure the % of aggregates, i.e. HMW degradants within the sample. The peak area percent (% area) and repeatability for the mAb and degradants (both HMW and LMW) were summarized in Table 2. Comparison of the % area showed a difference (Δ) of 0.02% for the HMW and the monomer, and %RSD’s within 3% for all analytes. The LMW produced higher %RSD, due to the low % area (0.05%), on both systems with some improvements on the ACQUITY Arc Bio System over the Agilent 1260 Infinity Bio-inert System.
The same SEC method was run on an ACQUITY Arc Bio System equipped with a Waters 2489 tunable UV detector (TUV) to explore the impact of UV detector types on data repeatability and sensitivity. The flow cells in UV detectors from Waters, including both TUV and PDA, use a TaperSlit design to allow all photons to reach the photodiodes to increase the sensitivity.4,5 Due to differences in optics design, the noise and drift of TUV detection is generally lower than that of PDA detection.4,5 Thus, TUV is known to have better sensitivity than PDA. The Waters 2998 PDA Detector uses a fixed slit width to establish 1.2 nm resolution across the diodes to give high quality UV spectra, while the Waters 2489 TUV Detector has a larger slit width (≤5 nm) to allow more light into the flow cell.
The TUV detector on the ACQUITY Arc Bio System produced a similar SEC chromatogram as the PDA for trastuzumab under the same method conditions. The data repeatability (n=3) of peak area % of HMW and LMW peaks for the TUV and the diode array detectors is shown below (Table 3). Analyses of the data show lower %RSDs for the TUV acquired data as compared to the PDA acquired data, especially for the HMW and LMW degradants.
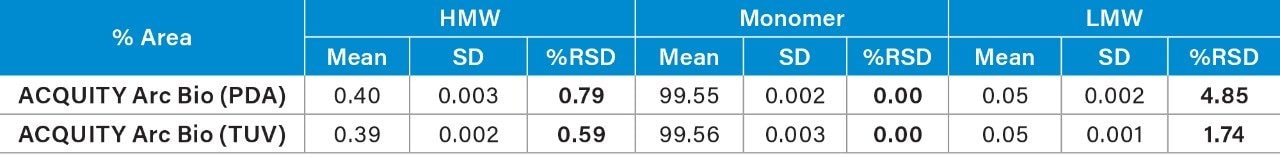
The USP s/n of the separation was also calculated using Empower Software for the detectors (Table 4). The same time segment in the chromatogram that is free of peaks was used for the noise calculation. Although the UV detectors investigated here provided adequate s/n for quantification, the TUV (2489) detection produced higher s/n. Improved sensitivity of TUV might contribute to the improvement of repeatability.
An SEC method of trastuzumab was transferred across an Agilent 1260 Infinity Bio-inert System and an ACQUITY Arc Bio System. The difference in retention times across the systems were within 0.1 min. The repeatability of the method was acceptable on both systems with the ACQUITY Arc Bio System exhibiting improvement for both the HMW and LMW degradants. The quantification of % area of low amount aggregates is reproducible across these two systems. The SEC method was also used to evaluate the effect of UV detector types, specifically tunable UV detector versus photodiode array detector. The results show tunable UV detector provided better data repeatability and higher sensitivity.
720006364, September 2018