
For forensic toxicology use only.
This application brief describes a simple UPLC-MS/MS method for the analysis of cTHC in preserved oral fluid at low pg/mL concentrations that can be used for confirmation analysis to support various forensic or roadside drug testing schemes.
A simple, sensitive UPLC-MS/MS method for the determination of cTHC in oral fluid collected using the Quantisal Oral Fluid Collection Device.
The requirement to analyze drugs at low levels in oral fluid has become an important requirement for many forensic laboratories around the world. The use of oral fluid as a biological matrix for forensic and roadside testing has significantly increased in popularity over the last decade. The presence of parent drugs in oral fluid can provide a better indication of current impairment or intoxication than when measured in urine, and for some drug substances the levels in oral fluid represent a convenient marker for circulating levels in blood. Oral fluid collection is a non-invasive technique and can be achieved without the privacy and adulteration issues associated with urine collection and, in contrast to blood samples, oral fluid does not require medically trained staff to collect the sample. The Quantisal Oral Fluid Collection Device (Immunalysis, USA) allows 1 mL of sample to be collected into a stabilizing buffer which promotes stability of the sample during transportation to the testing laboratory.
Cannabis is the most widely used illicit substance in the world and long-term use can lead to dependency. Cannabinoids are one of the most commonly detected classes of illegal drugs; consequently their analysis is of key importance in forensic testing.
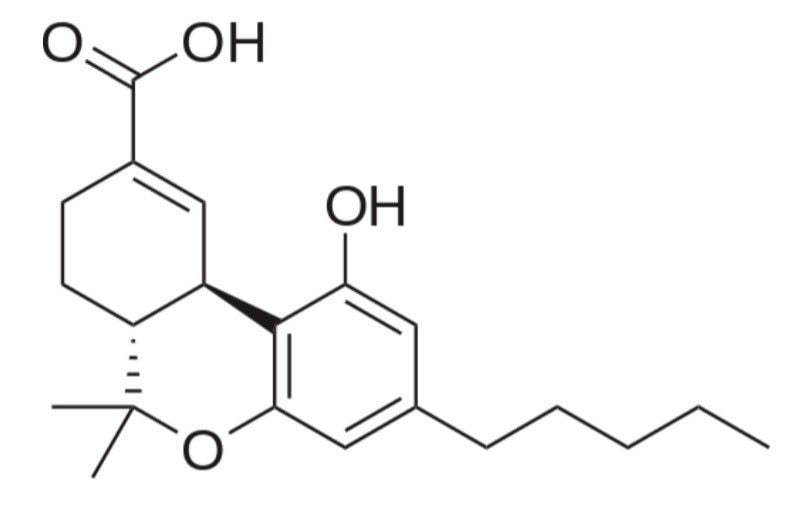
Δ-9-tetrahydrocannabinol (THC) is the major psychoactive element present in the plant Cannabis sativa and produces a number of metabolites including 11-nor-9-carboxy-Δ-9- tetrahydrocannabinol (cTHC). The advantage of measuring cTHC in oral fluid is that cTHC is not detected in cannabis smoke and hence documents active cannabis consumption. Monitoring this metabolite is therefore important as it helps differentiate active cannabis intake from passive environmental cannabis smoke exposure. However analysis of this metabolite is challenging as it is typically only found at low pg/mL concentrations; thus high sensitivity analytical techniques are required.
Control oral fluid was collected and preserved using the Quantisal Oral Fluid Collection Device according to the manufacturer’s directions. It is generally understood that the collected oral fluid is diluted by a factor of four once it has been added to the buffer in the device, and the concentrations stated in this technical brief relate to those in neat oral fluid. Once collected the samples were stored at 4 °C for at least 24 hours prior to analysis.
Control preserved oral fluid was spiked at a concentration equivalent to 20 pg/mL in neat oral fluid. A liquid-liquid extraction method was used to extract cTHC from the samples. Following evaporation of the organic layer which contains the cTHC, the sample was reconstituted in 50% acetonitrile.
The ACQUITY UPLC I-Class FTN was fitted with a 50 µL extension loop, which allowed 25 µL of sample to be analysed; cTHC was separated using a water/acetonitrile gradient on a CORTECS C18 Column.
Two MRM transitions were monitored for cTHC i.e., 343.2 > 245.2 (quantifier) and 191.1 (qualifier). The internal standard (cTHC-d3) was monitored using the transition 346.2 > 248.2.
In this technology brief the Xevo TQ-S micro was used in conjunction with the ACQUITY UPLC I-Class System to detect cTHC at very low concentrations.
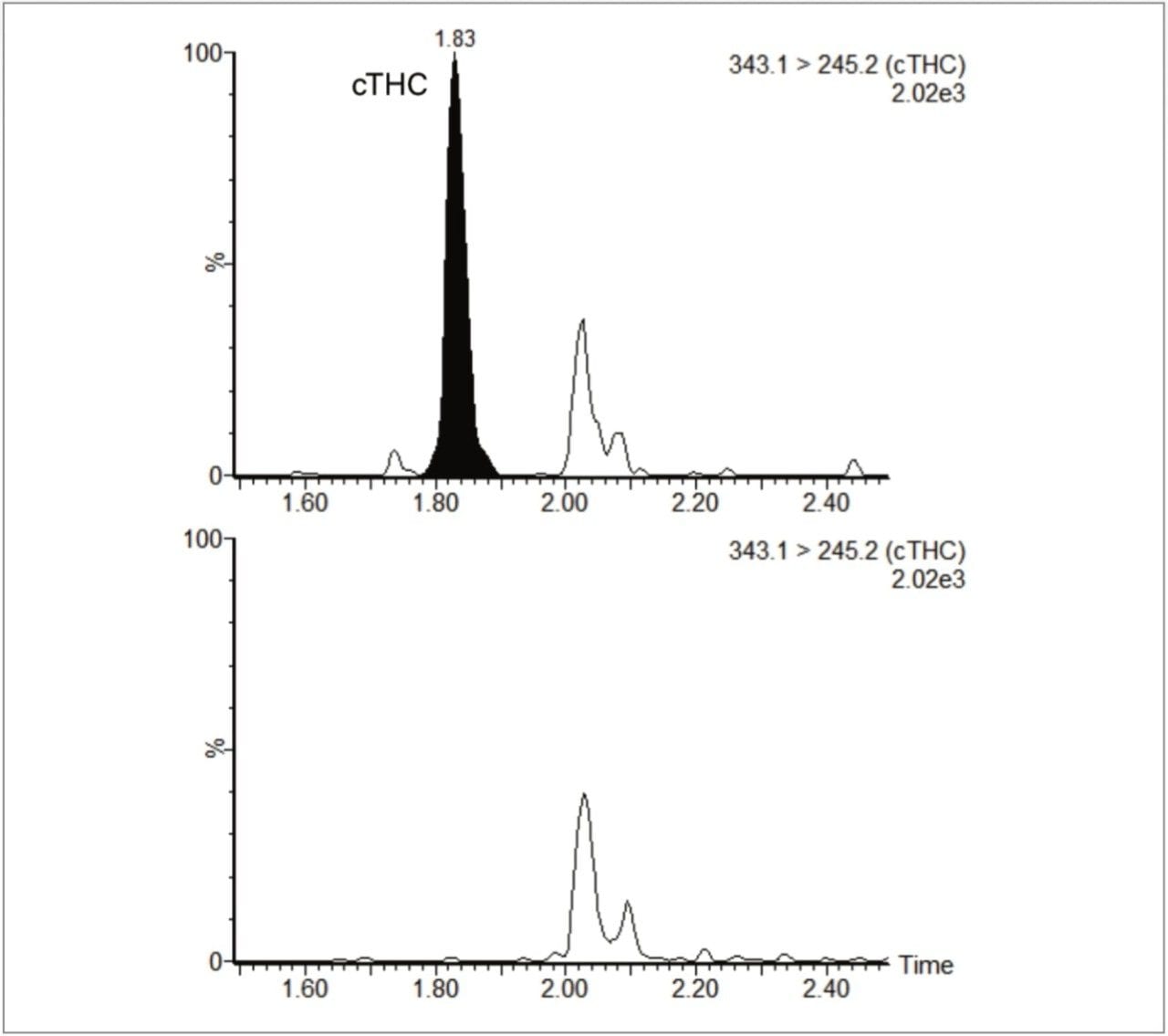
A comparison of a control (blank) preserved oral fluid extract with a spiked preserved oral fluid extract is shown in Figure 1. The figure shows the smoothed and integrated quantifier MRM trace for both samples.
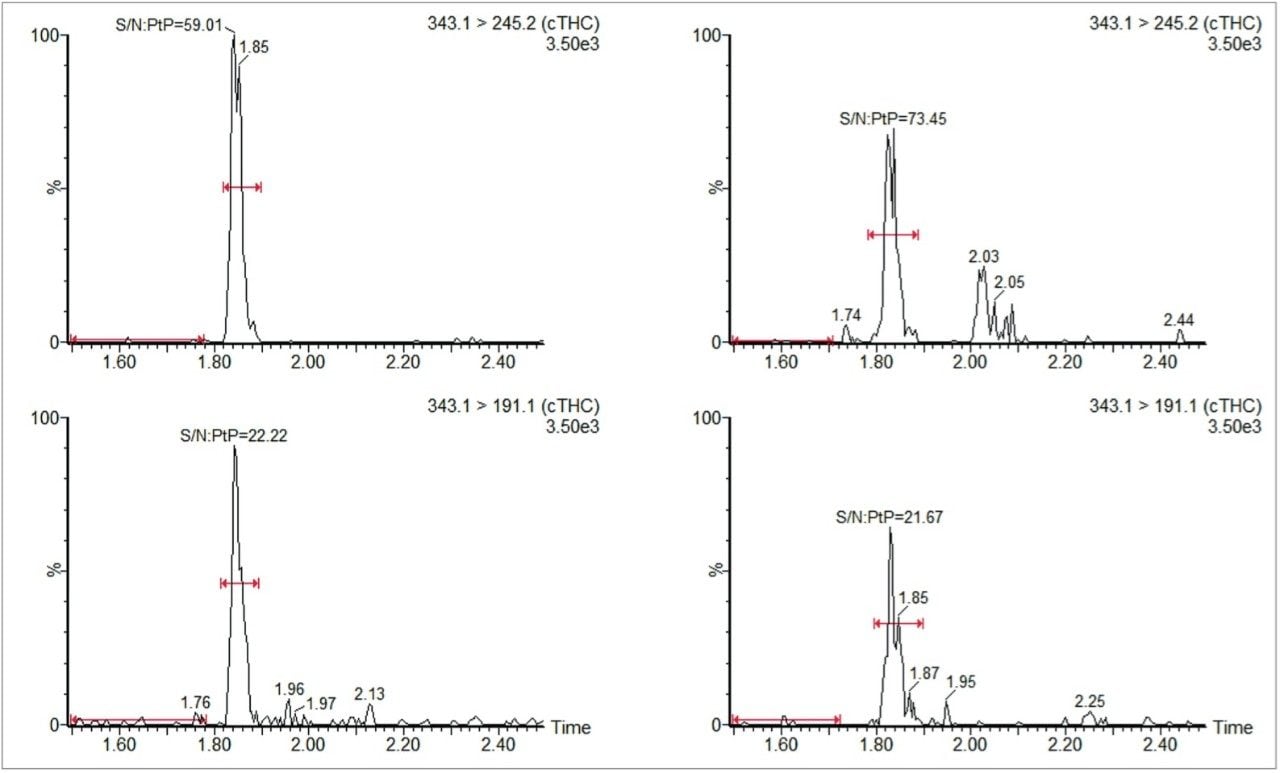
The signal-to-noise (peak-to-peak on unsmoothed raw data) for both MRM transitions in the spiked sample is shown in Figure 2 along with those for an equivalent concentration matrix-free standard.
The increasing use of oral fluid in forensic and roadside drug testing has highlighted the need for a quick, sensitive, accurate, reliable, and robust method to quantify illicit drugs in this frequently used biological matrix. The use of preserved oral fluid allows for simple, supervised and non-invasive collection of a matrix which contains analytes commonly measured in such testing schemes.
The Xevo TQ-S micro has demonstrated the required analytical sensitivity to detect cTHC in preserved oral fluid at low pg/mL concentrations.
720005944, March 2017