This is an Application Brief and does not contain a detailed Experimental section.
This technology brief demonstrates the capabilities of Empower software in combining multiple 2D acquisition channels collected during a single injection to create one result for analysis of impurities.
Combine multiple acquisition channels collected during a single injection to generate one result for analysis of impurities with Empower Chromatography Data Software.
Mass spectrometry provides sensitivity, selectivity, and rich-mass information not only to support small-molecule drug development, but also for routine monitoring of low-level impurities in the finished drug products. While different acquisition modes are available with mass spectrometry, the single ion recording (SIR) acquisition mode provides much better sensitivity compared to the total ion chromatogram (TIC) mode. The SIR measures intensity of the single ion of interest and simplifies analysis for the targeted compounds. Often, sample components may have different masses and multiple channels with different SIR values must be collected for one sample injection. These multiple channels must be combined to create one result.
This technology brief illustrates use of Empower Software for combining multiple MS SIR channels for analysis of potential genotoxic impurities (PGIs). Alkyl esters of benzenesulfonic acid are considered PGIs that can develop during the synthesis of a drug substance and must be accurately measured at low levels to ensure safety of the pharmaceutical products.
Empower Software enables users to combine multiple MS SIR channels acquired for a single injection. To illustrate this capability, we analyzed PGIs of benzenesulfonic acids methyl, ethyl, and isopropyl esters (MBS, EBS, IBS) that have different masses.
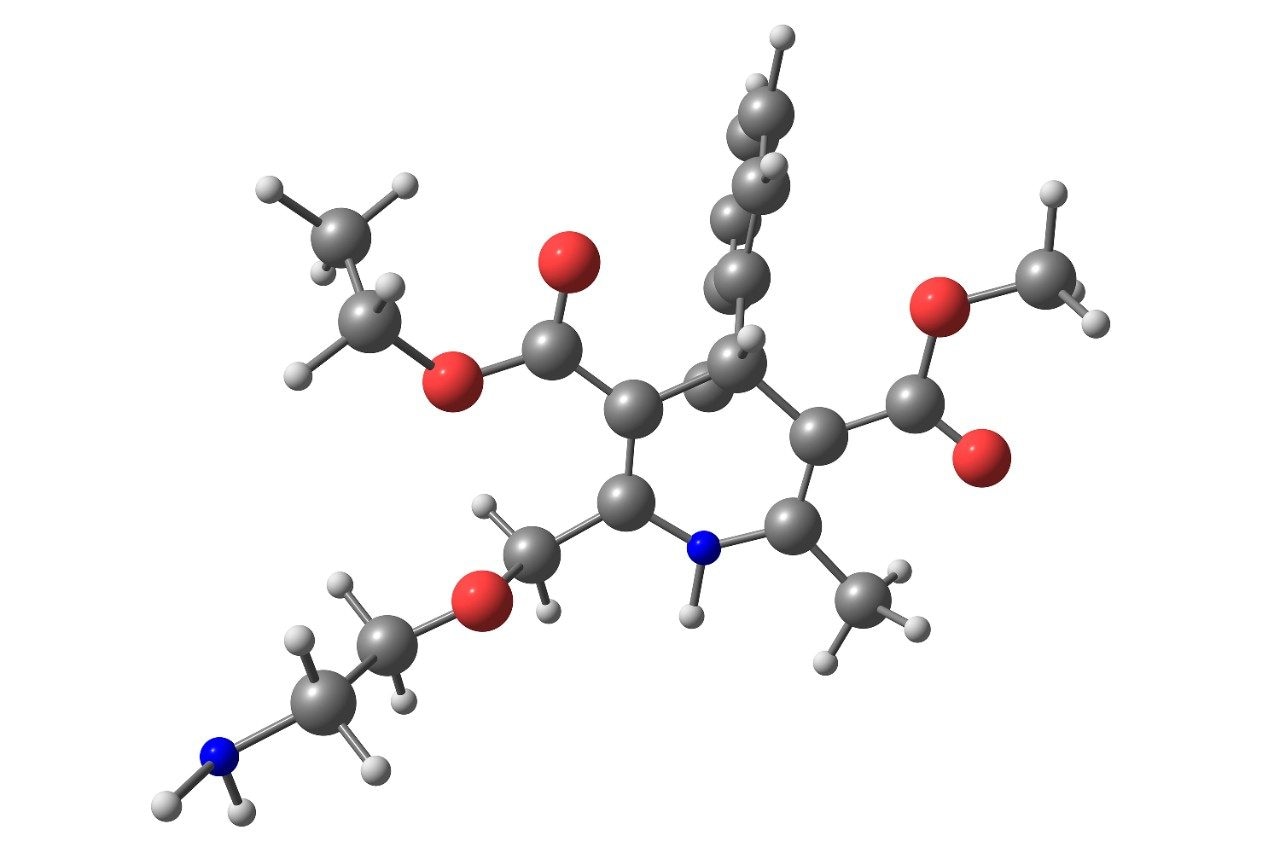
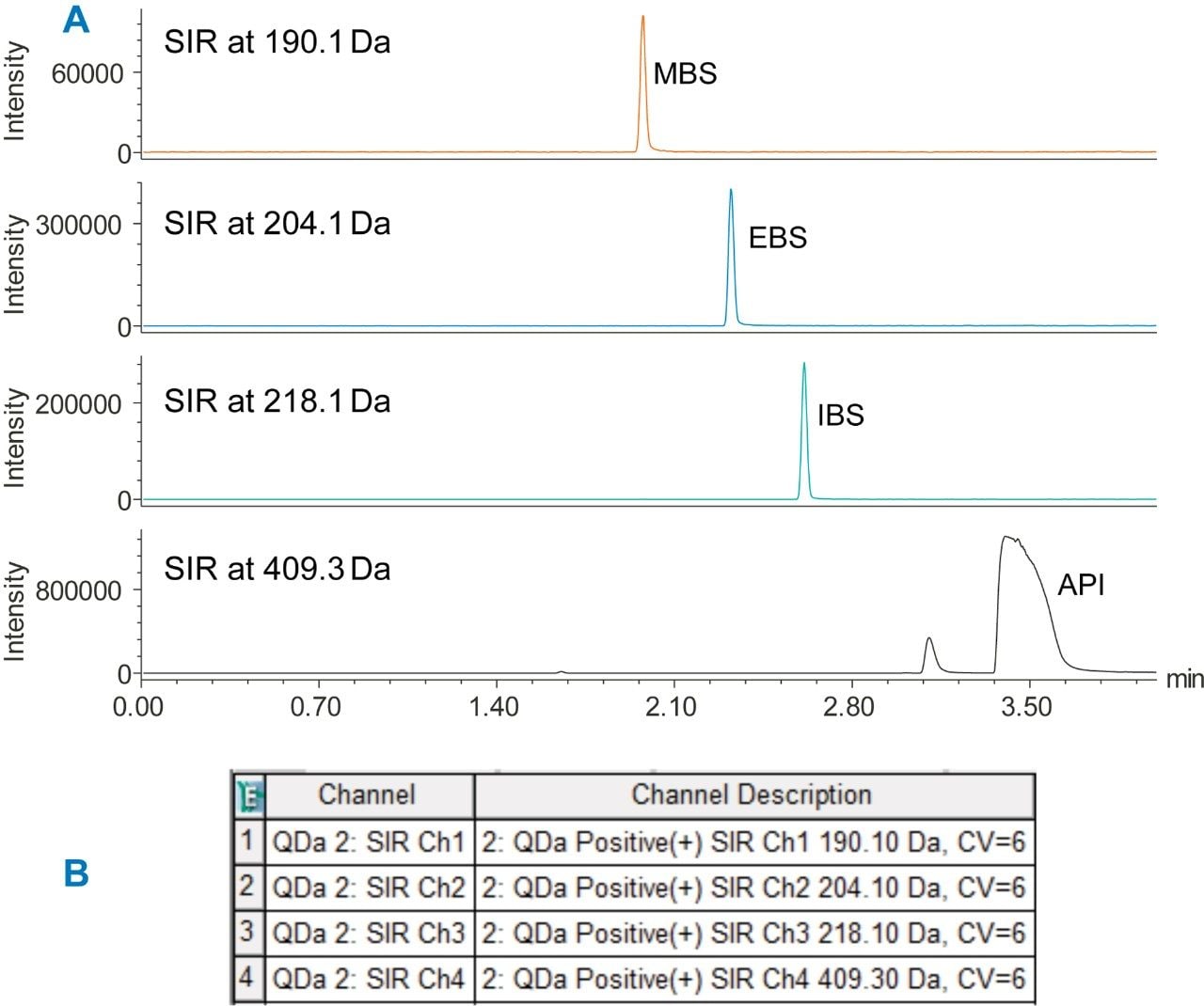
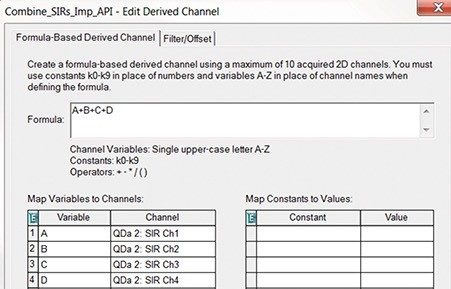
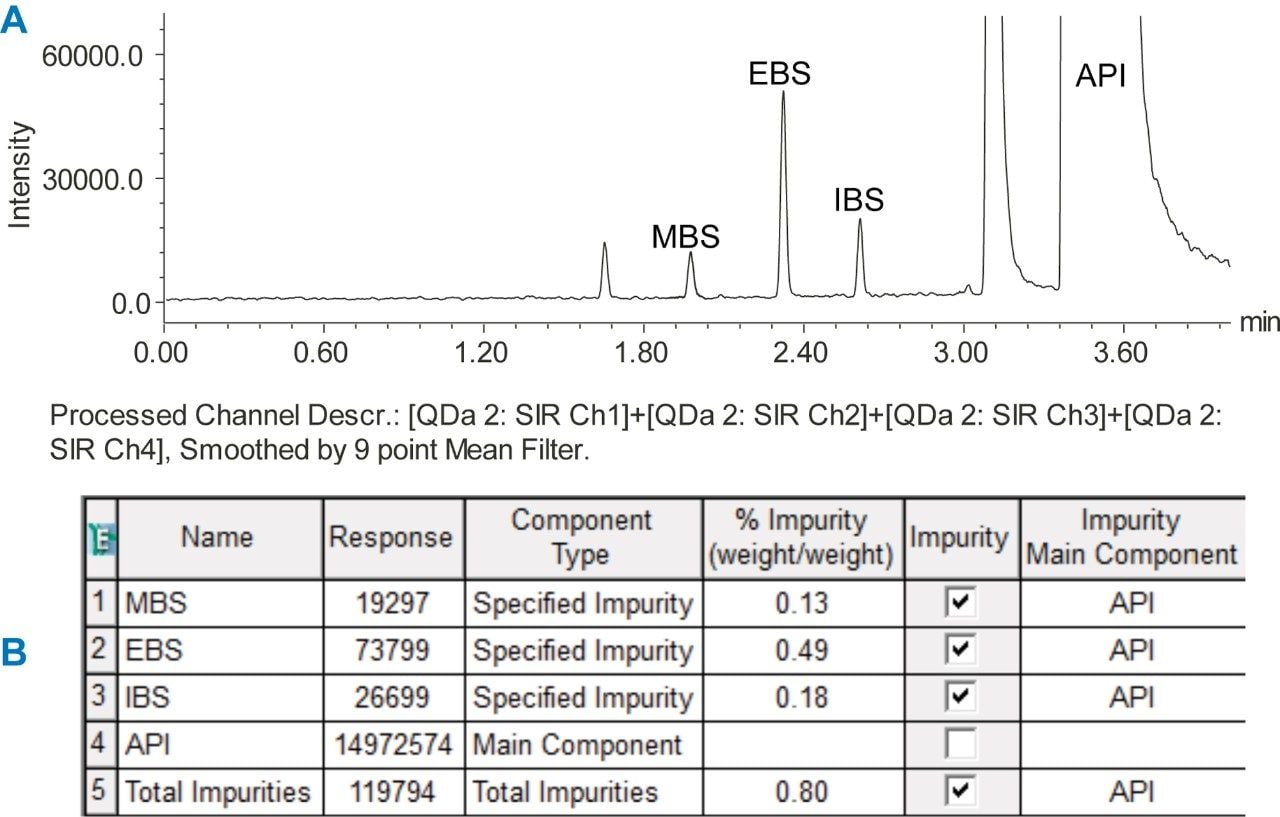
A sample solution containing 0.1 mg/mL of amlodipine besylate active pharmaceutical ingredient (API) was spiked with the esters at 0.1% level and analyzed by an ACQUITY QDa Detector using previously developed UPLC method.1 Four SIR channels were acquired to collect data for the esters and the API (Figure 1). Then, we created a formula-based derived channel to combine the SIR channels (Figure 2) and processed the data. Empower Software generated one chromatographic plot with each ester and API (Figure 3A). The combined data was then used to determine quantity of each impurity in the API sample injection (Figure 3B).
Empower 3 Software enables users to combine multiple 2D acquisition channels collected for a single injection to generate one sample result.
This capability can be easily adapted by any laboratory that utilizes mass spectrometry and a single ion recording (SIR) acquisition mode for low-level analysis of impurities with different masses. Multiple channels with different SIR values can be combined to determine quantity each impurity in one sample injection. This tool will streamline quantitative analysis protocols during development of the drug substance or release testing of the finished drug products.
720006127, November 2017