
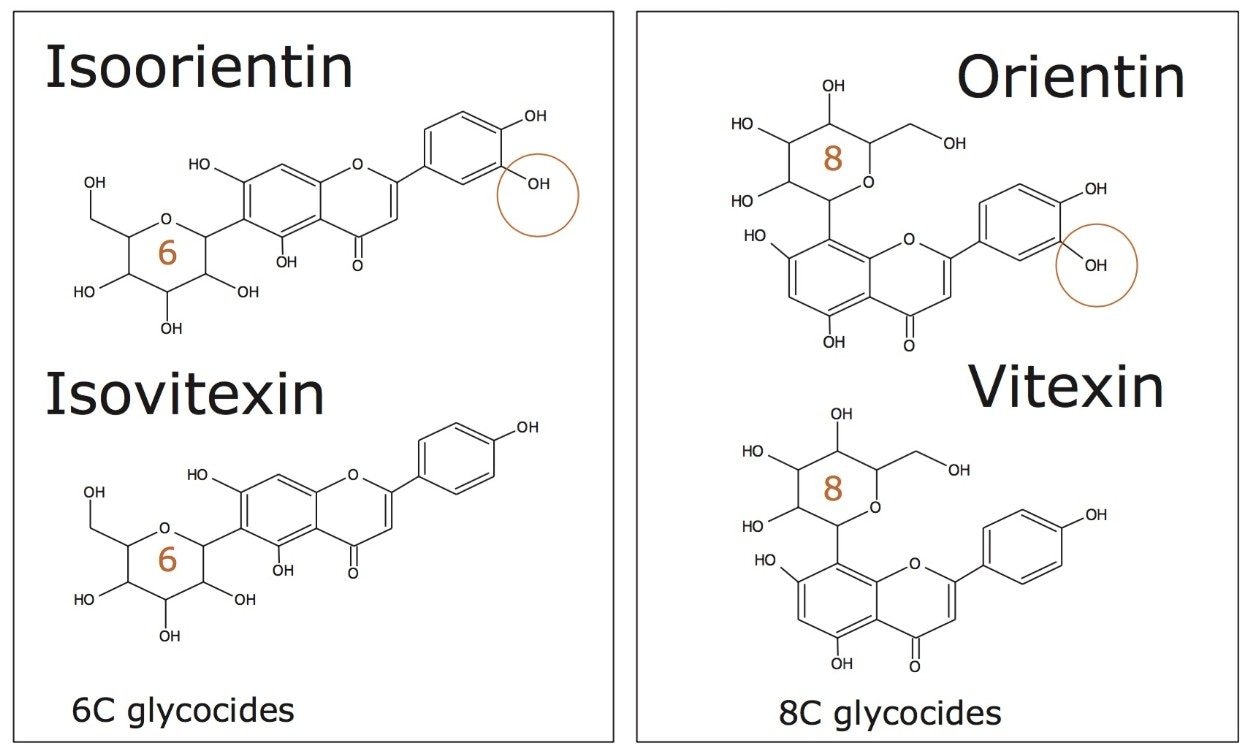
The genus Passiflora is comprised of approximately 450 species, but only a few are commercially exploited. Several Passiflora (Passifloraceae) species are utilized as phytomedicines (anxiolytics). These contain flavonoids, mainly C-glycosylflavones- apigenin and luteolin derivatives which frequently occur as isomers.
Flavonoids are polyphenolic compounds that are ubiquitous in nature and are one of the largest and most widespread classes of compounds. To date, over 4,000 flavonoids have been identified and they have attracted considerable interest due to their diverse pharmacological and biological properties. Such attributes mean many flavonoid containing plant species may be used as functional foods or phytomedicines.
The most well-known species of this family is Passiflora incarnata, which is found in Europe and North America. Originally from Brazil, P.edulis is very popular and cultivated in many countries for its edible fruits. Passion fruit (P.edulis and P.alata) is known as Maracujá in Brazil, the largest producer of P.edulis in the world. In the U.S. and EU it is popular because of its pleasant taste and sedative/traquilizing effects from infusion of its leaves. Leaf extracts are also utilized for flavoring and in juices by the food industry. The fruit are used in juices, ice creams, or eaten naturally.
Passiflora edulis is also native to tropical areas of other South American countries, including Paraguay, Argentina, and Peru. In addition to its anxiolytic effects P.edulis leaves are recognized for their antiinflammatory activity. The sedative properties of the Passiflora species are related to its phytochemical makeup.
It is therefore important that consumer products be authenticated with confidence, and that an accurate profile of the phytochemical composition is obtained.
The use of flavonoid compounds as markers to profile a species or authenticate a consumer product is important, but also a challenge due to sample complexity. Analysis of such complex samples can be difficult when the sample is comprised of isomers. Subtle changes in chromatography resulting from the sample matrix, column loading, and chromatographic conditions can result in the coelution of isomers.
In this application note, we investigate the use of UPLC with ion mobility mass spectrometry as a route to specific and unambiguous identification of flavonoid isomers. UPLC-IM-MS offers unique advantages to profiling complex mixtures. We demonstrate a routine, non-targeted screening workflow that can uniquely determine the presence of these isomeric flavonoid markers. The UPLC-IM-MS system enables the authentication of phytochemical makeup with unmatched specificity that cannot be provided by mass accuracy alone.
The combined peak capacity of UPLC-IM-MS and nitrogen buffer gas, travelling wave collision cross section measurements (TWCCSN2) can be used for routine unequivocal identification of marker flavonoid isomers in complex mixtures such as herbal tea products and dietary supplements. The genus Passiflora is comprised of approximately 450 species, but only a few are commercially exploited. Several Passiflora (Passifloraceae) species are utilized as phytomedicines (anxiolytics). These contain flavonoids, mainly C-glycosylflavones- apigenin and luteolin derivatives which frequently occur as isomers. Flavonoids are polyphenolic compounds that are ubiquitous in nature and are one of the largest and most widespread classes of compounds. To date, over 4,000 flavonoids have been identified and they have attracted considerable interest due to their diverse pharmacological and biological properties. Such attributes mean many flavonoid containing plant species may be used as functional foods or phytomedicines.
In the IM-MS authentication study performed P.incarnata, P.edulis, P.caerulea, and P.alata species have been profiled. The most well-known species of this family is Passiflora incarnata, which is found in Europe and North America. Originally from Brazil, P.edulis is very popular and cultivated in many countries for its edible fruits. Passion fruit (P.edulis and P.alata) is known as Maracujá in Brazil, the largest producer of P.edulis in the world. In the U.S. and EU it is popular because of its pleasant taste and sedative/traquilizing effects from infusion of its leaves. Leaf extracts are also utilized for flavoring and in juices by the food industry.2 The fruit are used in juices, ice creams, or eaten naturally. Passiflora edulis is also native to tropical areas of other South American countries, including Paraguay, Argentina, and Peru.
In addition to its anxiolytic effects P.edulis leaves are recognized for their anti-inflammatory activity. The protective role that the intake of P.edulis aqueous extract might exert in an organism has been studied. The phytochemical analysis of P.edulis leaves contained twice the flavonoid content of P.alata.3 A consumer product containing P.edulis will therefore have a greater phytochemical content that will produce greater sedative effects than a product containing an equivalent amount of P.alata. If P.alata. was substituted for P.edulis, the desired and expected sedative effects may not be experienced. Products labeled as “Natural” are not neccessarily safe, but such products can be, if produced to legisative standards. Manufacturers have to prove that their products have been made to strict standards and contain a consistent and clearly marked dose, as stipulated in EU Directive 2004/24/EC, which came into full effect 30 April 2011.
The sedative properties of the Passiflora species are related to its phytochemical makeup. It is therefore important that consumer products be authenticated with confidence, and that an accurate profile of the phytochemical composition is obtained. The use of HPLC-MS and HPLC-MS/MS based methods to profile flavonoids has become more routine. The use of flavonoid compounds as markers to profile a species or authenticate a consumer product is important and also a challenge due to sample complexity. Zucolotto et. al.,4 performed an analysis of C-glycosyl flavonoids from the South American Passiflora species, where vitexin was not identified in Passiflora edulis, as was the case for Pereira et. al.5 However utilizing UPLC and time-of-flight (Tof) MS, the presence of vitexin in P.alata was confirmed.6 Analysis of such complex samples can be difficult when the sample is comprised of isomers. Subtle changes in chromatography resulting from the sample matrix, column loading, and chromatographic conditions can result in the coelution of isomers. Even using mass spectrometry alone, with MS/MS or CID, it is difficult to determine true identification, especially when the isomers produce the same fragment or product ions. However Pereira et. al. reported distinctive fragmentation patterns produced for 6-C and 8C glycocides,7 while March et. al.8 further confirmed these observations. Since the phytochemical makeup can affect biological properties, further advances in analytical techniques to profile flavonoids could impact the design of functional food products by providing additional information about their health and safety benefits, as well as improved taste.
In this application note, we investigate the use of UPLC-IM-MS as a route to specific and unambiguous identification of flavonoid isomers, as this technique offers some unique advantages to profiling complex mixtures. It is a combination of high resolution mass spectrometry and high efficiency ion mobility based measurements and UPLC separations. Ion mobility is a rapid orthogonal gas separation phase technique that allows another dimension of separation to be obtained within an LC timeframe. Compounds can be differentiated based on size, shape, and charge.
|
UPLC system: |
ACQUITY UPLC I-Class |
|
Column: |
ACQUITY UPLC BEH C18, 100 mm x 2.1 mm, 1.7 μm |
|
Column temp.: |
45 °C |
|
Flow rate: |
0.75 mL/min |
|
Mobile phase A: |
H2O (0.1% Formic acid) |
|
Mobile phase B: |
Acetonitrile (0.1% Formic acid) |
|
Time (min) |
Flow rate |
%A |
%B |
|---|---|---|---|
|
0.00 |
0.75 |
98.0 |
2.0 |
|
1.00 |
0.75 |
98.0 |
2.0 |
|
5.00 |
0.75 |
95.0 |
5.0 |
|
10.00 |
0.75 |
80.0 |
20.0 |
|
13.00 |
0.75 |
70.0 |
30.0 |
|
15.00 |
0.75 |
20.0 |
80.0 |
|
15.10 |
0.75 |
98.0 |
2.0 |
|
17.00 |
0.75 |
98.0 |
2.0 |
|
Injection volume: |
2 μL |
|
MS system: |
SYNAPT G2-Si |
|
Ionization mode: |
ESI - |
|
Capillary voltage: |
2.3 kV |
|
Sample cone voltage: |
20 V |
|
Desolvation temp.: |
600 °C |
|
Lockmass and LockCCS: |
Leucine enkephalin, [M-H]- =554.2620 |
|
Acquisition range: |
50 to 1200 m/z |
|
Acquisition rate: |
10 spectra/sec |
|
Collision energy ramp: |
35 to 75 eV |
|
Resolution: |
20,000 FWHM (res mode) |
|
Default IMS parameters: |
IMS T-Wave velocity ramp: |
|
|
Start: 1000 m/s |
|
|
End: 300 m/s |
|
IMS T-Wave pulse height: |
40 V |
|
IMS gas flow: |
90 mL |
Hydroethanolic extracts P.incarnata, P.edulis, P.caerulea, and P.alata
Extraction with ethanol-water (2:1 v/v, 1 g plant/10 mL solvent according to Brazilian Pharmacopoeia procedure). SPE cleanup (Sep-Pak. RP-18, elution with 60% methanol – H2O).
Hydromethanolic fraction=4 mg/mL.
Hydromethanolic fraction dilution using H2O: 10:1 (400 μg/mL) and 40:1 (100 μg/mL).
A collision cross section (CCS) value is a robust and precise physicochemical property of an ion. It is an important distinguishing characteristic that is related to its chemical structure and three-dimensional conformation.8 UsingNitrogen buffer gas, travelling wave collision cross section measurements (TWCCSN2) measurements can help increase targeted screening specificity. Previously generated TWCCSN2 measurements have been entered into the UNIFI Scientific Library. This allows the expected and determined TWCCSN2 values to be utilized to screen and confirm the presence of isomeric flavonoid markers. Here we present TWCCSN2 values (derived from ion mobility drift times) as a new identification parameter, which can be used to distinguish flavonoid isomers and to profile unknowns.
TWCCSN2, molecular ion accurate mass, ion mobility product ions, and retention time have been used to profile the hydromethanolic extracts of P. incarnata, P. alata, P. edulis, and P. caerulea, that were grown in Brazil. Results obtained clearly show the benefits of using CCS measurements and the combined peak capacity of the ACQUITY UPLC I-Class System with ion mobility. The enhanced peak capacity enabled more information to be extracted from product ion studies, and the individual ion mobility product ion spectra have been obtained for isomeric flavonoid isomers, which were coeluting. From the extracts characteristic assignment for 6-C and 8-C flavonoid glycoside isomers (vitexin/isovitexin) (orientin/isoorientin) were obtained, this is not possible using mass resolution alone.
In Figure 2 the complexity of the P.edulis extract is illustrated by the UPLC-IM-MS electrospray negative mode conventional base peak ion chromatogram obtained. However in Figure 3, using the ion mobility Data Viewer in UNIFI, the true complexity of the P.edulis sample profiled using ion mobility separation orthogonal to UPLC separation is shown.
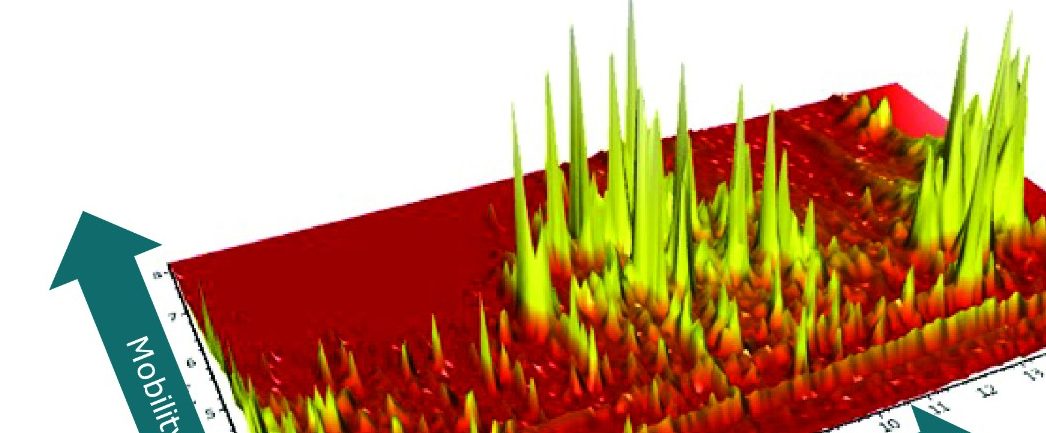
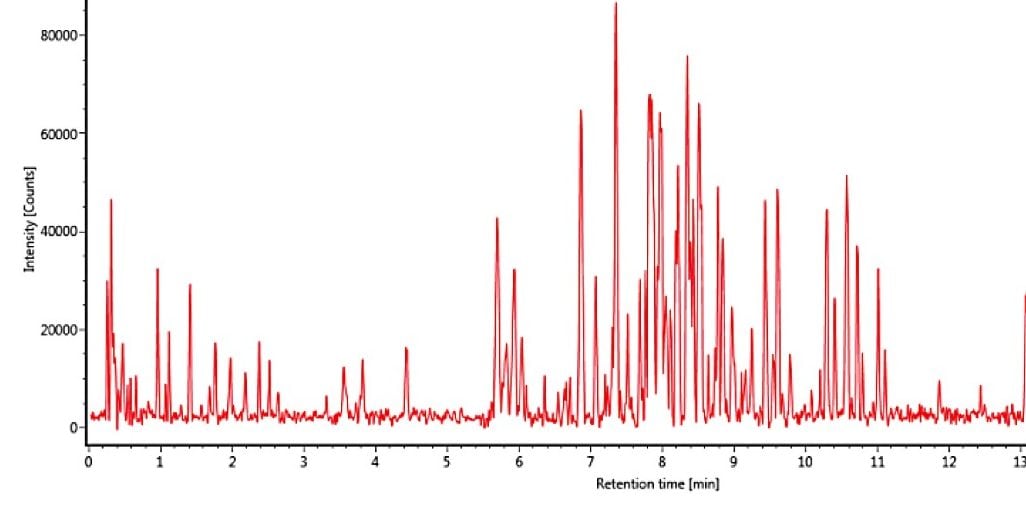
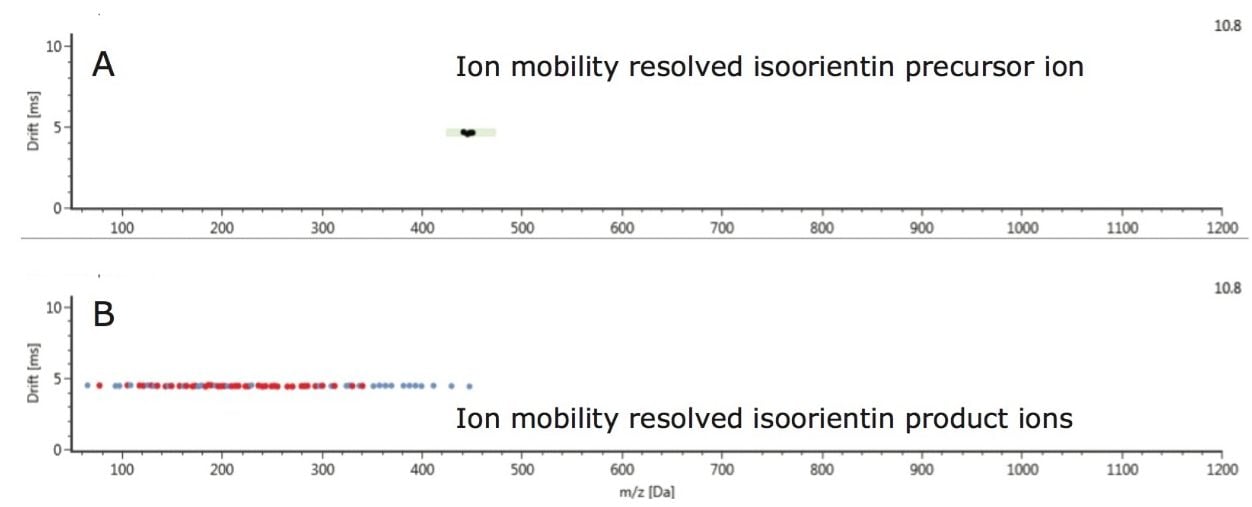
The enhanced peak capacity not only allows chromatographically coeluting compounds to be resolved, but also isomers. This enables their respective single component precursor/ion mobility product ion spectra to be generated and adds a high degree of specificity to the collision induced fragmentation spectra produced, during this generic screening acquisition process. The ion mobility product ions are aligned with their precursor ions using both retention time and ion mobility drift time. Hence two analytes at the same retention time, but at a different drift times, result in ion mobility product ion spectra which are completely separated from each other. It is this process that allows the cleanest ion mobility product ion spectra to be produced.
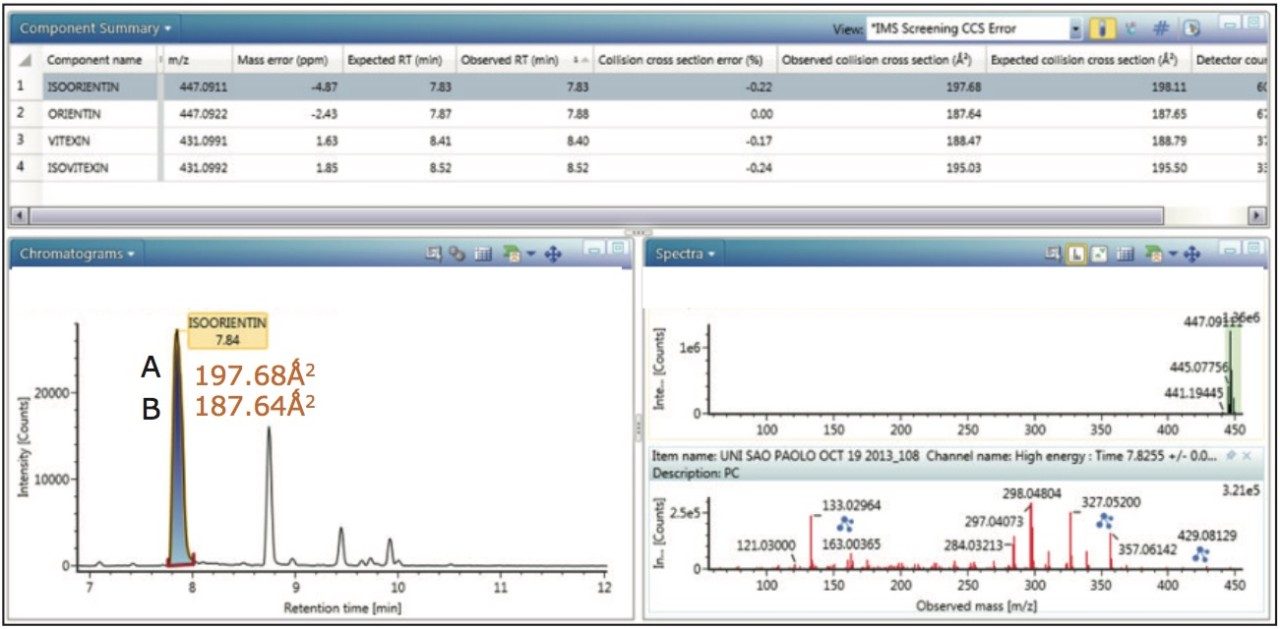
CCS measurements were obtained for the marker flavonoid standards, and this information was used to create a scientific library in UNIFI incorporating the expected TWCCSN2 values. The four Passiflora extracts were routinely screened against the UNIFI flavonoid TWCCSN2 library to determine the presence and unequivocal identification of the 6-C and 8-C flavonoid glycosides isomers. Expected TWCCSN2 measurements for the flavonoid markers, isoorientin (197.68Å2), orientin (187.64Å2), vitexin (188.47Å2), and isovitexin (195.03Å2), were determined using standards. It is notable, that for each respective isomer pair, the 8-C-glycoside has a smaller collision cross section, and this can be used as a relative distinguishing characteristic. The observation made further illustrates how the selectivity of TWCCSN2 can be utilized. If retention times shift due to matrix, or if alternative chromatography methods are used, the glycoside pairs could still be distinguished regardless of retention time.
TWCCSN2 measurements for marker glycoside pairs (vitexin/isovitexin) 188.8Å2/195.5Å2 have been determined, in the complex extract of Passiflora caerulea, shown in Figure 4, with respective CCS errors of -0.17% and -0.24%, when compared to the expected CCS values within the flavonoid screening library in UNIFI.
Also illustrated in Figure 4 is the UNIFI Component Summary for Passiflora caerulea, which shows the selected vitexin/isovitexin extracted mass chromatogram, with the corresponding precursor and ion mobility product ion spectra obtained. Mass measurement errors <2 ppm are presented, along with expected/observed collision cross sections and collision cross section errors. Additionally the extracted mass chromatogram shows the presence of a series of isobaric components between 8.0 and 12.0 minutes.
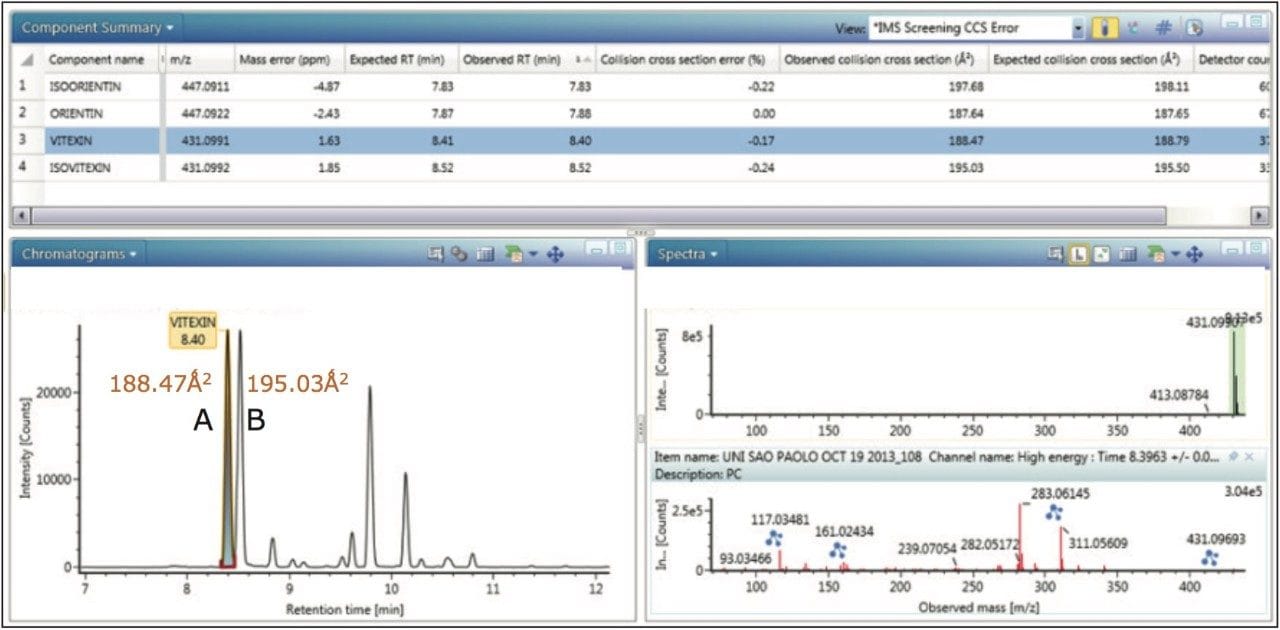
For the marker glycoside pair (orientin/isoorientin), 187.64Å2/197.68Å2 has been determined with respective CCS errors of 0% and 0.22%. For all four species profiled, the CCS and mass measurement errors determined are shown in Table 1. The reproducibility and robustness of TWCCSN2 measurements are further illustrated in Table 2, where the assay was repeated 10 months later as part of a TWCCSN2 multivariate analysis study. When the results were compared to the expected TWCCSN2 values in the UNIFI screening library created 10 months earlier, errors of <0.3% were obtained.
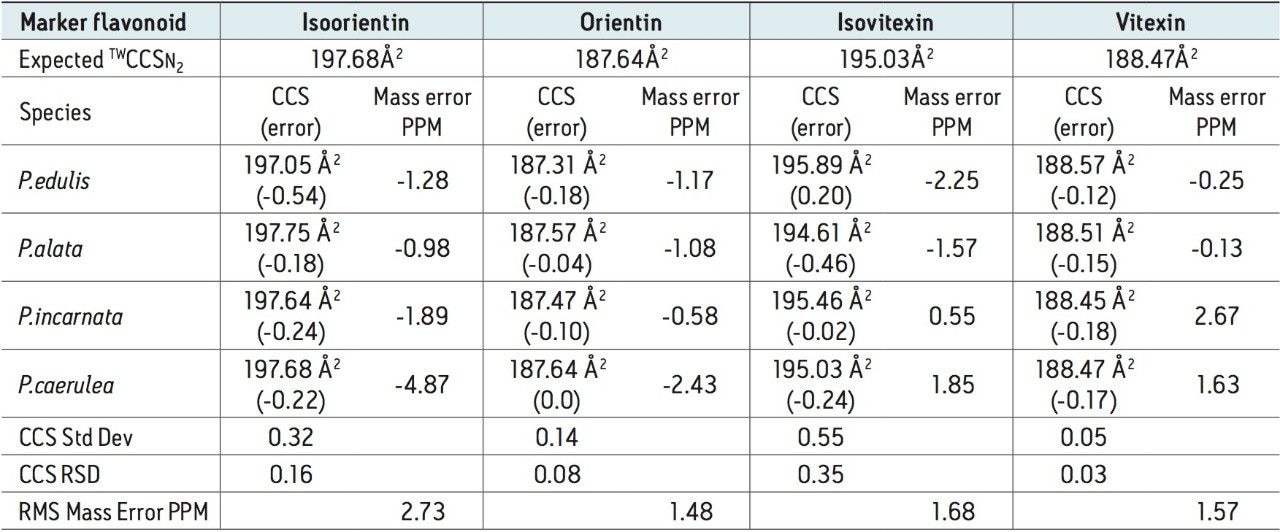
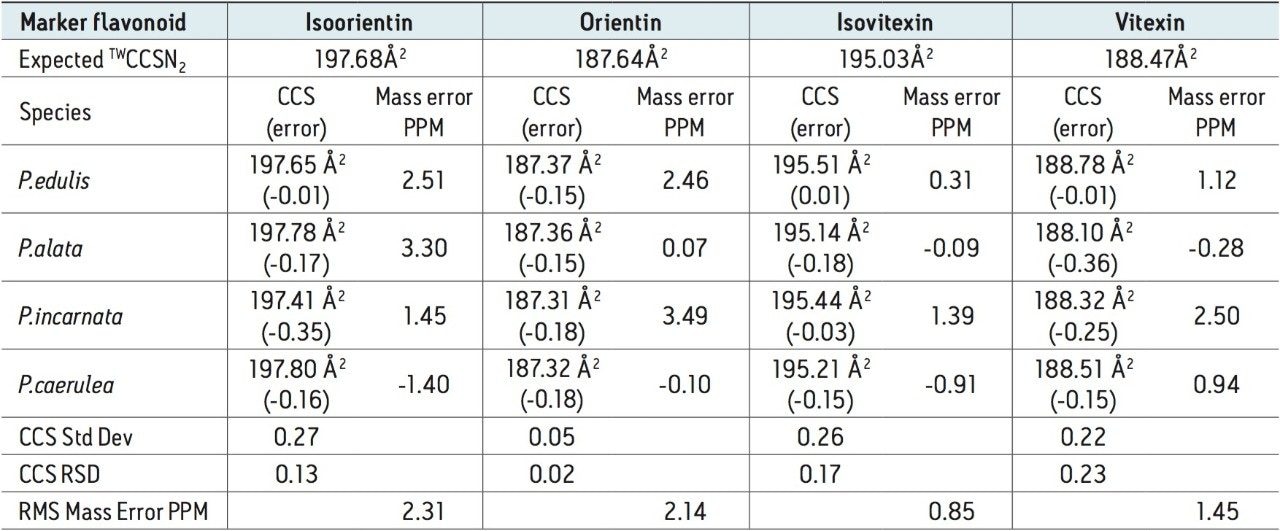
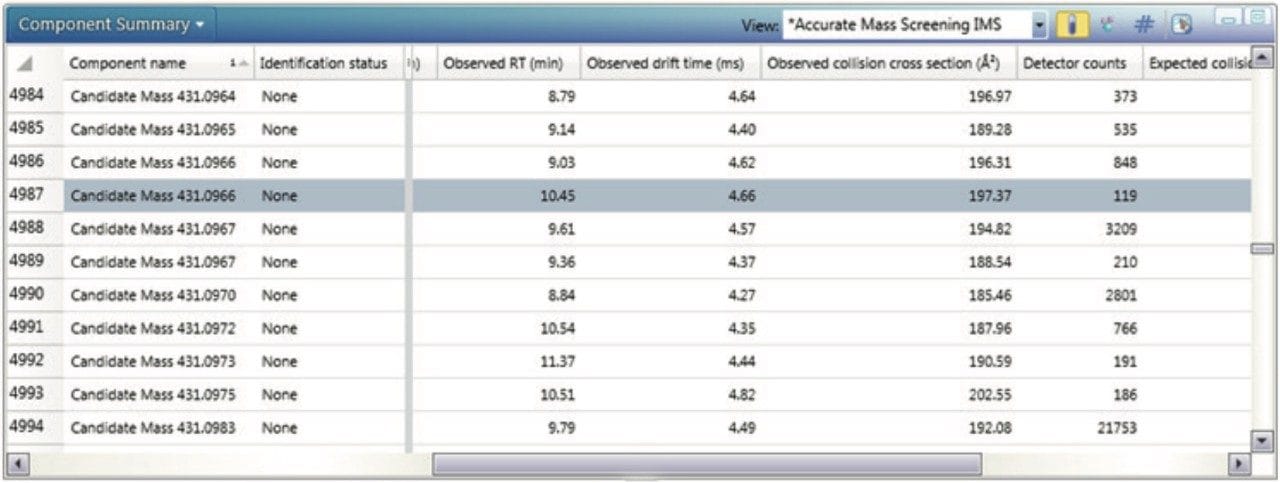
The corresponding UPLC-IM-MS drift time plot (ion mobility resolution) versus retention is shown in Figure 5. Here, the true complexity of the sample profiled is illustrated, where separation orthogonal to UPLC separation is obtained using ion mobility. The series of isobaric components between 8.0 and 12.0 minutes can be differentiated using TWCCSN2. The unique, patented UNIFI mobility data viewer allows the displayed data to be saved as a bookmark, that can later be used to rapidly manually interrogate/investigate all of the Passiflora species profiled. The TWCCSN2 values obtained are presented in the UNIFI Component Summary of isobaric candidates in Figure 6, where the observed retention time, observed collision cross section, observed drift time, and detector counts are shown for the ion mobility separated species. The information obtained can then be added into the analysis method to target compounds of interest.
For Figure 7 the UPLC-IM-MS drift time plot versus retention time for Passiflora alata is shown. The ion mobility separation profile for vitexin/isovitexin is presented, it can be seen that these two isomeric marker flavonoids have been determined to be present in Passiflora alata, where they can be detected and differentiated using TWCCSN2 screening with ion mobility mass spectrometry.
Both vitexin/isovitexin were determined at the expected retention time with accurate mass measurement error of <5ppm and a collision cross section error of <0.5%. In previous studies, vitexin was not determined to be present in Passiflora alata but technology enhancements have enabled this identification to be made here. Previous studies using HPLC presented a small shoulder on the base of the chromatographic peak of isovitexin but even with accurate mass measurement, it was not possible to identify the vitexin peak since the species were isomeric. Also there was not sufficient sensitivity to identify the unique fragmentation profile of vitexin.
Further studies with enhancements in sensitivity and the use of the ACQUITY UPLC I-Class System, enabled vitexin to be partially chromatographically resolved and detected. However, the challenge remained to chromatographically resolve both pairs of marker glycosides. In this study where UPLC is combined with ion mobility separation, it was possible to develop a method that exploited the separation power of both techniques. UPLC parameters provide optimum separation of isovitexin and vitexin, while ion mobility parameters provide optimum separation of isoorientin and orientin. Orientin and isoorientin were not chromatographically resolved, but were resolved with ion mobility. This example shows how the combined peak capacities of chromatography and ion mobility can be used to provide the desired separation.
The benefits of ion mobility extend to reducing spectral complexity, as shown in Figure 8, where the conventional retention time aligned multi-component precursor and fragment ion spectra, for a chromatographic peak vitexin (m/z 431.0983) generated at retention time 8.395 mins is shown. In addition to m/z 431.0983, many other ions are present in the spectrum.
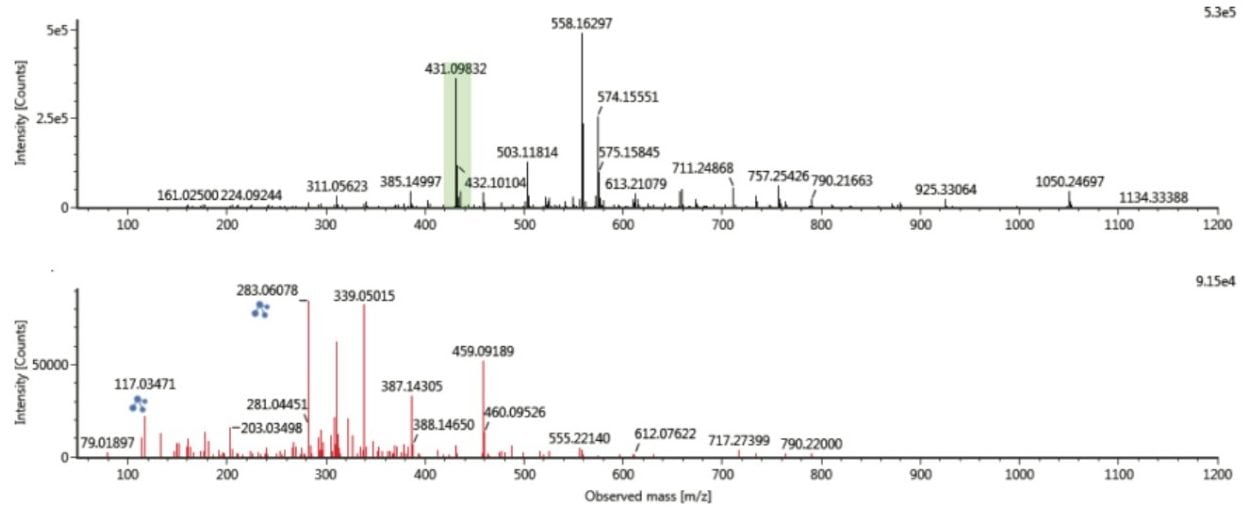
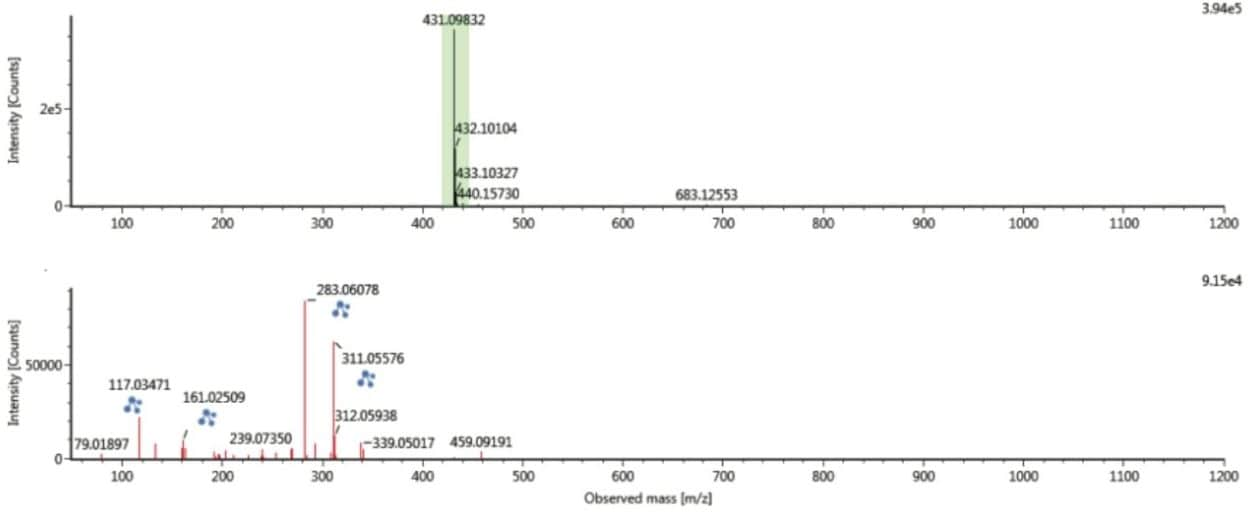
However as can be seen in Figure 9, when the spectrum is derived at both retention time and drift time, the chromatographically coeluting ions are mobility resolved and the spectrum is “cleaned up” for both the precursor and ion mobility product ions. The result is a highly specific ion mobility product ion spectrum. Such specific product ion spectra are generated for all analytes detected in the sample analyzed, including unknowns and non-targeted compounds.
The UNIFI Component Summary for Passiflora caerulea, illustrating chromatographically coeluting isoorientin and orientin selected in the extracted mass chromatogram window, with the corresponding isoorientin precursor and ion mobility product ion spectra obtained. The 6C/8C glycoside pair orientin/isoorientin differ in retention time by 1.24 seconds. However, when analyzing real extracts, there is a small difference in retention time and the concentrations vary from species to species. In this study, we saw that the targeted species merge to form one peak. Mass measurement errors <5ppm and CCS errors <0.5% are presented. The extracted mass chromatogram also shows the presence of a series of isobaric components between 7.0 and 11.0 minutes.
For marker glycoside pair (orientin/isoorientin), 187.65Å2/197.68Å2 has been determined with respective CCS errors of 0% and 0.22%. From Figure 11 the UPLC-IM-MS of drift time plot versus retention time for Passiflora caerulea is presented. Here, the ion mobility separation profile for chromatographically coeluting isoorientin (peak marker illustrated) and orientin has been selected and automatically extracted using the Zoom to Component feature of the UNIFI Software.
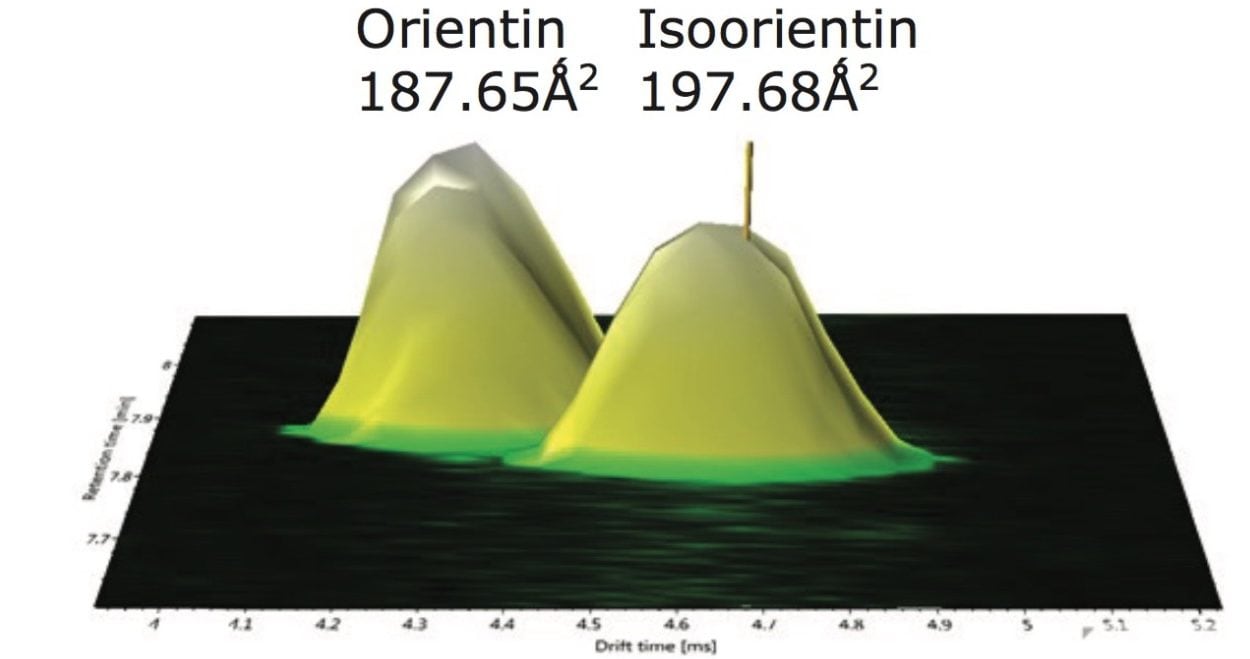
The results obtained demonstrate that it is possible to distinguish the marker isomer pairs for the extracts analyzed using TWCCSN2 measurements. When comparing the expected and the measured collision cross sections, the TWCCSN2 measurement errors were typically <0.5%. In addition, it has been possible to acquire the “cleaned up” ion mobility product ion spectra, that are mobility resolved from coeluting components. In the case of isoorientin/orientin (which coeluted chromatographically and had the same product ions), ion mobility resolution enabled ion mobility product ion ratios to be observed. For the first time, unique collision cross section measurements and corresponding isomer fragmentation spectra have been obtained.
Figures 12 and 13 show the resolved ion mobility product ion spectra for isoorientin and orientin (chromatographically coeluting) determined to be present in Passiflora caerulea. Both isomers produce the same product ions, however it can be seen that if the correct collision energy profile is applied, unique product ion ratios can be induced for the individual isomers.
The ion mobility product ion spectra and routes of the 6C and 8C glycosides have previously been elucidated.4 The unique ion mobility product ion ratios at m/z 284/285 and m/z 297/298/299 can be used to characterize the isomer pair, in conjunction with unique TWCCSN2 measurements. In the case of isoorientin, the product ion ratios are m/z 284 < m/z 285, and m/z 297 <m/z 298 >m/z 299. Whereas for orientin, the ion mobility product ion ratios are m/z 284 >m/z 285, and m/z 297 >m/z 298 <m/z 299. For isovitexin and vitexin the unique product ion ratios at m/z 281/282/283/284 can be used to characterize the isomer pair in conjunction with unique CCS measurements. In the case of isovitexin, the ion mobility product ion ratios are m/z 281 >m/z 282 >m/z 284. Whereas for vitexin, the ion mobility product ion ratios are m/z 281 >m/z 282 <m/z 284. In both cases the ion mobility product ion m/z 283 forms the base peak ion of the characteristic product ion clusters.
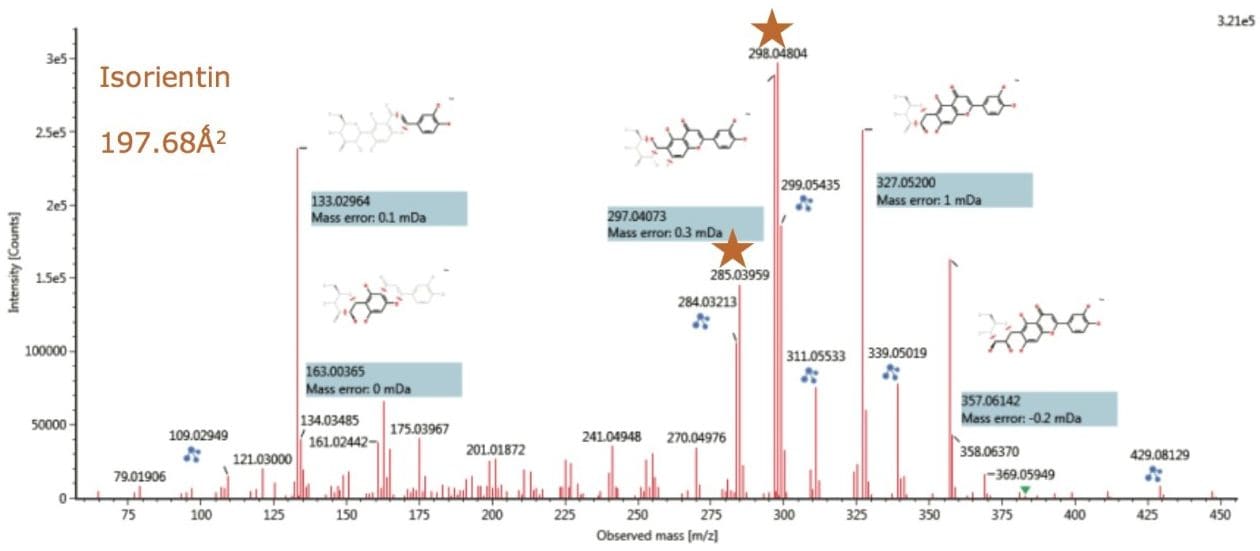
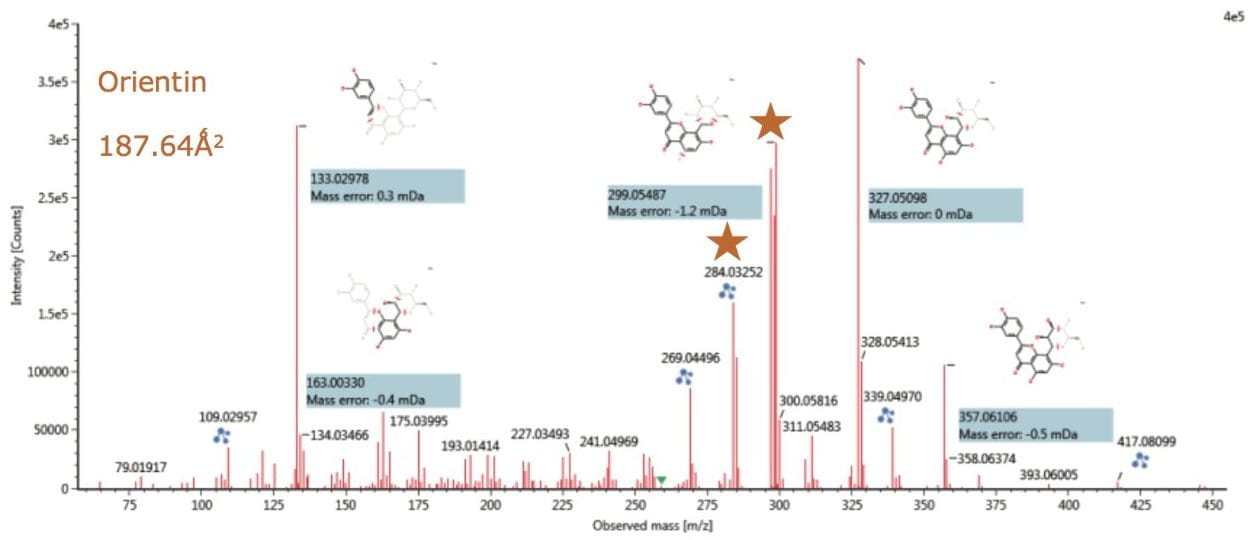
Also it can be seen that the proposed fragmentation pathway can be determined using UNIFI Software, where accurate mass measurement <5 ppm has been routinely obtained. The enhanced peak capacity produced in IMS experiments enables the fragmentation spectra produced to be resolved from coeluting structurally related flavonoids present in the extract, allowing this characteristic information to be used with confidence.
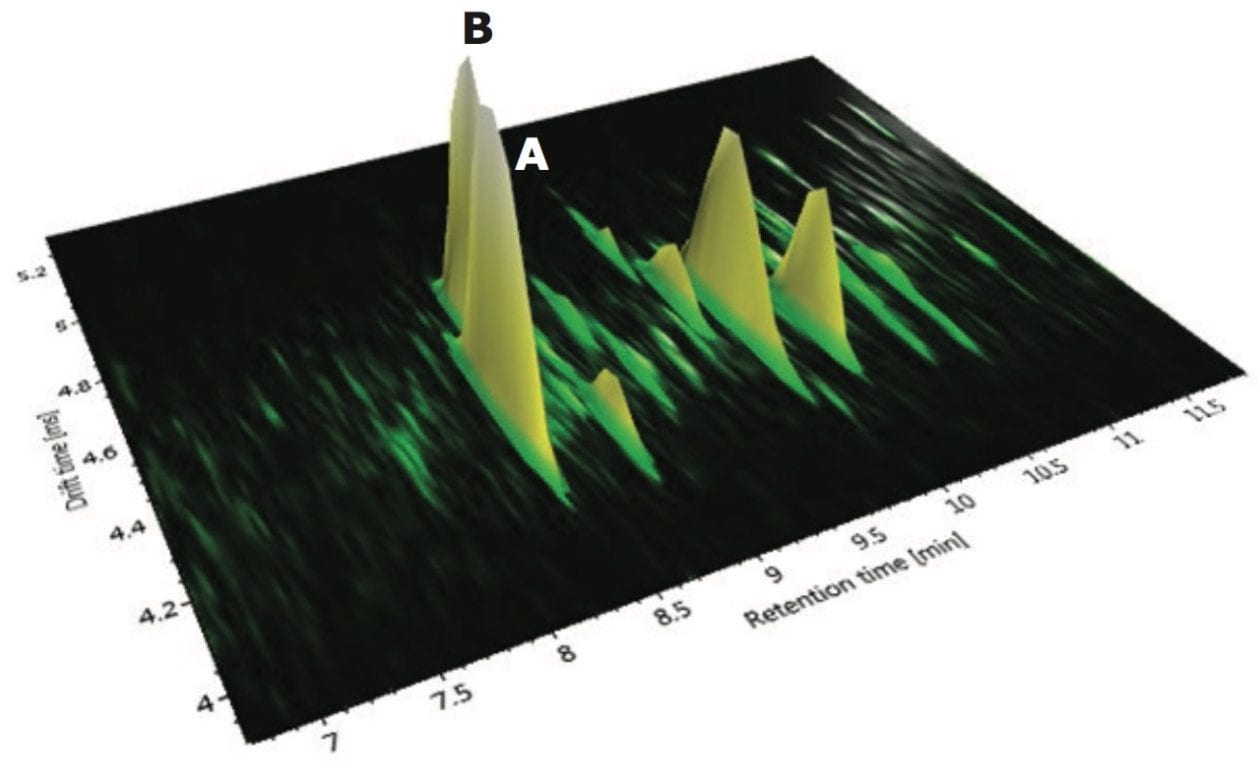
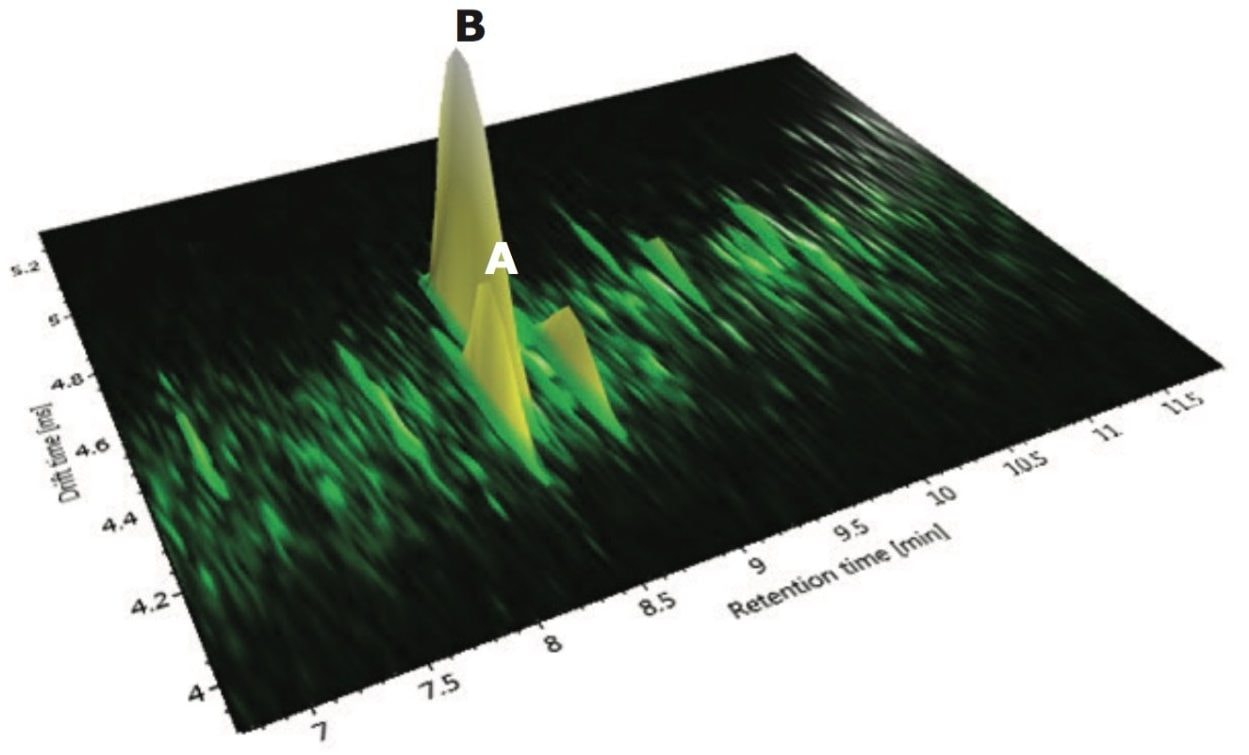
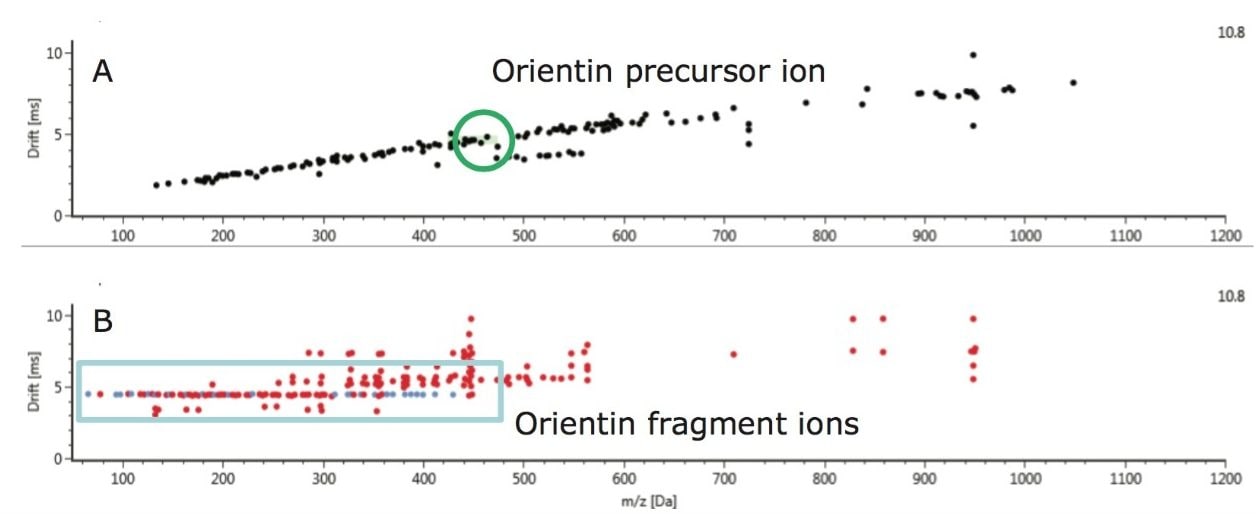
The corresponding ion mobility spectral resolution obtained, where the ion mobility drift plots for m/z versus drift time can be seen, is presented in Figures 14 and 15. In Figure 14, drift plot A shows all the ions detected at retention time 7.83 minutes, and drift plot B shows the corresponding retention time-aligned fragments produced from the precursor ions that are chromatographically coeluting. The observed precursor ion of isoorientin is automatically highlighted in green, and the corresponding expected fragment ions are shown in blue in plot B. UNIFI Software has been specifically designed to intelligently visualize ion mobility data. From Figure 15, it can be seen that it is possible to select only the observed precursor ion and the ion mobility product ions being produced at a specific m/z and drift time. In the case of isoorientin observed in Passiflora caerulea, the ion mobility drift plot for all of the ions detected at retention time 7.83 minutes and drift time 4.67 milliseconds can be seen.
Commercially pioneered by Waters, MSE ion fragmentation takes advantage of the duty cycle and benefits of time-of-flight technology. This has facilitated the acquisition of accurate fragment ion information, allowing the analyst to obtain structural elucidation and confirmation information for 10,000’s of compounds in a single acquistion. HDMSE ion mobility product ion formation further enhances the specificity of retention time aligned MSE data acquisition through the addition of drift time alignment. Using ion mobility separation it is possible to isolate the precursor ions and also those ions produced during the collision induced dissosciation process. HDMSE ion mobility product ion acquisition offers an unparalleled approach to bringing the specificity of MS/MS to all compounds detected in an acquisition. As can be seen from Figures 12 and 13, HDMSE ion mobility product ion acquisitions can provide greater selectivity than conventional MS/MS.
720005424, June 2015
FAQs about UPLC-IM-MS
What is UPLC-IM-MS and how does it enhance specificity and identification in authentication profiling of natural food products?
UPLC-IM-MS stands for Ultra-Performance Liquid Chromatography-Ion Mobility-Mass Spectrometry. It is a powerful analytical technique that combines high-resolution separation, ion mobility, and mass spectrometry to enhance specificity and identification in authentication profiling of natural food products. It allows for the separation and identification of complex mixtures of compounds with high precision and accuracy.
How can UPLC-IM-MS be used in routine separation approaches for natural food product authentication?
UPLC-IM-MS can be used in routine separation approaches for natural food product authentication by providing a comprehensive analysis of the sample. It can detect and identify various compounds present in the sample, including contaminants, adulterants, and natural product markers. This helps in ensuring the authenticity and quality of the food product.
What are the advantages of using UPLC-IM-MS in food product authentication compared to traditional methods?
UPLC-IM-MS offers several advantages over traditional methods in food product authentication. It provides higher resolution and sensitivity, allowing for the detection of trace levels of compounds. It also enables the separation of isomeric compounds, which is challenging with conventional techniques. Additionally, UPLC-IM-MS provides faster analysis times and requires less sample preparation, making it a more efficient and cost-effective approach.
Can UPLC-IM-MS be applied to different types of natural food products?
Yes, UPLC-IM-MS can be applied to a wide range of natural food products. It is a versatile technique that can be tailored to analyze specific compounds or classes of compounds based on the characteristics of the food product. Whether it is fruits, vegetables, grains, spices, or beverages, UPLC-IM-MS can provide valuable insights into their composition and authenticity.
Are there any limitations or challenges associated with UPLC-IM-MS in food product authentication?
While UPLC-IM-MS is a powerful technique, it does have some limitations and challenges. One challenge is the complexity of data analysis, as the technique generates large amounts of data that need to be processed and interpreted accurately. Additionally, the availability of reference standards for all compounds of interest can be limited, which may affect the identification and quantification of certain compounds. However, advancements in data analysis software and the growing availability of reference standards are addressing these challenges.