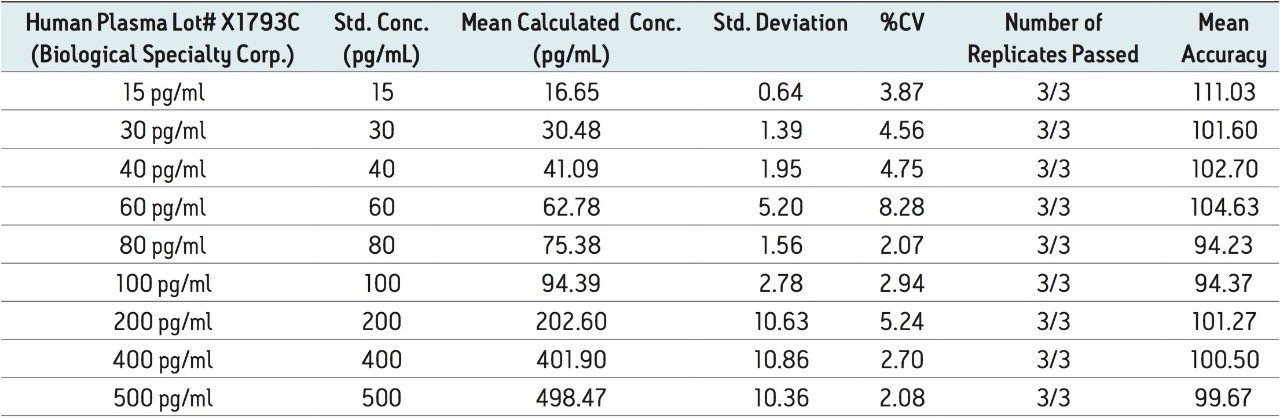
This study provides a single, simple method for the quantification of teriparatide in human plasma. The method uses UPLC and fast, selective sample prep in 96-well format to achieve a limit of detection (LOD) of 15 pg/mL, a dynamic range of 15 to 500 pg/mL, and an average QC accuracy of 97.5% (across six sources of matrix) from 200 μL of human plasma.
Teriparatide (FORTEO) is a recombinant form of a fragment of human parathyroid hormone, used in the treatment of osteoporosis. As a major global health problem, osteoporosis is responsible for 1.5 million bone fractures each year. Teriparatide is the first treatment for osteoporosis that actually stimulates new bone formation. It is an anabolic drug that acts to strengthen bones with the potential to improve skeletal micro architecture, thereby increasing bone density. In contrast to bisphosphonate drugs that treat osteoporosis by reducing or preventing bone loss, teriparatide promotes bone production.
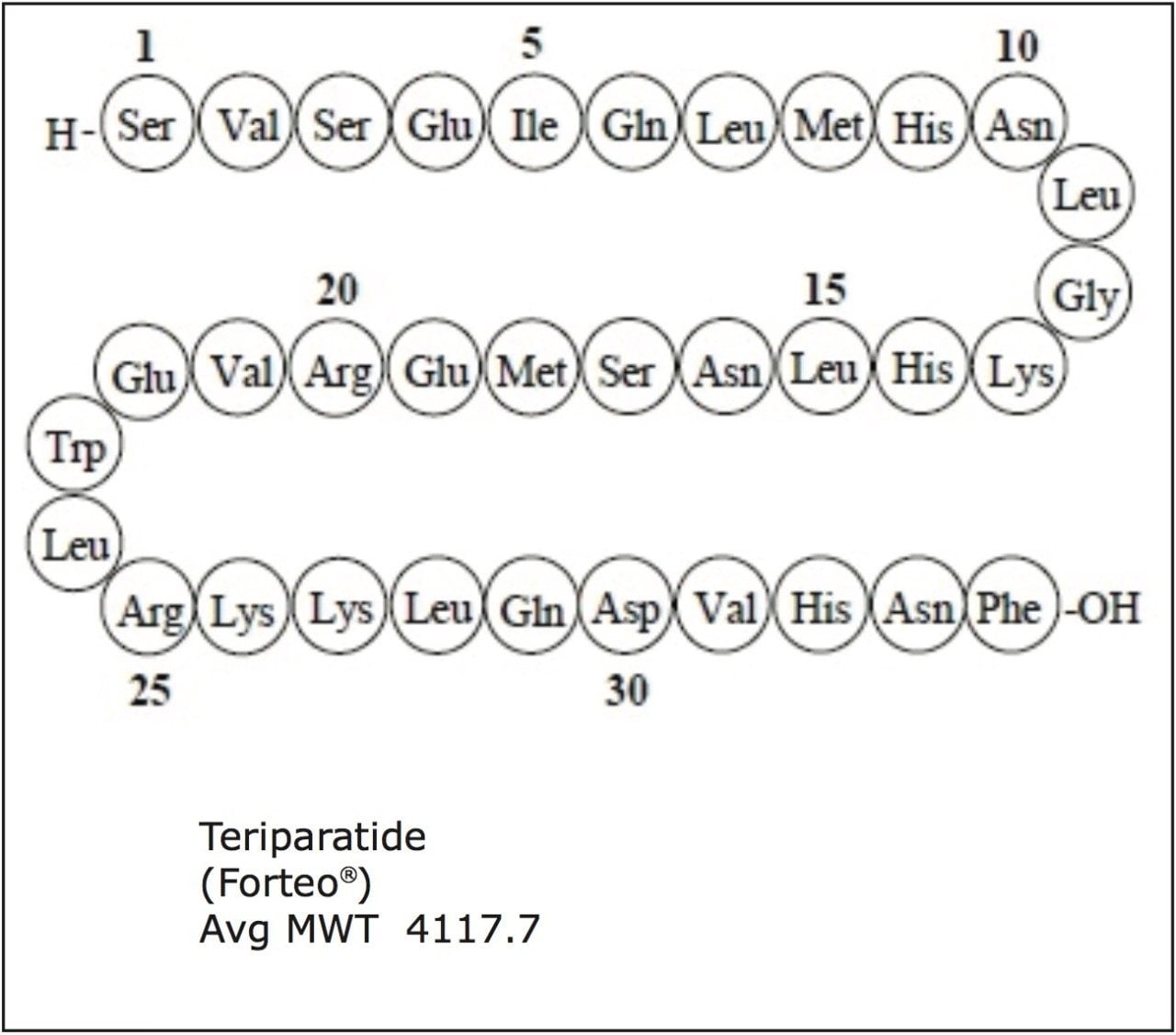
Teriparatide is comprised of the first 34 amino acids (the biologically active region) of the 84-amino acid human parathyroid hormone (PTH), also referred to as [rhPTH (1-34)].
Although biologics like teriparatide have historically been quantified using ligand binding assays (LBAs), recently there has been a trend toward the analysis of large molecules by LC-MS/MS. This is, in part, driven by the fact that LBAs can suffer from significant cross-reactivity issues and lack of standardization. LC-MS/MS offers the following benefits: shorter development times, greater accuracy and precision, the ability to multiplex, and readily distinguish between closely related analogues, metabolites, or endogenous interferences. Large peptides, such as teriparatide, are particularly difficult to analyze by LC-MS/MS, as MS sensitivity is low due to poor transfer into the gas phase and poor fragmentation. In addition, teriparatide suffers from significant non-specific binding and poor solubility, making LC and sample preparation method development challenging. The pharmacokinetics of teriparatide are characterized by rapid absorption within 30 minutes and rapid elimination with a half-life of one hour, resulting in a total duration of exposure to the peptide of approximately four hours. At the practical clinical dose of 20 μg, typical teriparatide levels are ~50 pg/mL, making detection by LC-MS/MS even more difficult. Traditional LC-MS/MS assays for teriparatide have involved time-consuming and laborious immunoaffinity purification and/or multidimensional or nano-flow LC. This study provides a single, simple method for the quantification of teriparatide in human plasma, as shown in Figure 1. The method uses UPLC and fast, selective sample prep in 96-well format to achieve a limit of detection (LOD) of 15 pg/mL, a dynamic range of 15 to 500 pg/mL, and an average QC accuracy of 97.5% (across six sources of matrix) from 200 μL of human plasma.
Step 1: Protein Precipitation (PPT): 20 μL of human parathyroid (1–38), which was used as an internal standard (2 ng/mL), was added to 200 μL of human plasma and mixed.
Samples were precipitated in a 1-mL 96-well plate with 200 μL acetonitrile containing 5% ammonium hydroxide, and centrifuged for 15 min at 4000 rpm.
The supernatant was transferred to a 2-mL 96-well plate containing 1 mL of water then mixed.
|
Step 2: |
Solid-phase extraction (SPE) using an Oasis HLB μElution 96-well plate (p/n 186001828BA) |
|
Condition: |
200 μL methanol |
|
Equilibrate: |
200 μL water |
|
Load sample: |
Entire diluted PPT supernatant (~1.4 mL) was loaded onto the extraction plate in two steps |
|
Wash: |
200 μL 5% methanol |
|
Elute: |
2 x 25 μL 60:34:5:1 acetonitrile/water/ trifluoroethanol/ trifluoroacetic acid |
|
Dilute: |
50 μL water |
|
Inject: |
30 μL |
|
System: |
ACQUITY UPLC |
|
Column: |
ACQUITY UPLC CSH C18, 2.1 x 50 mm, 1.7 μm (p/n 186005296) |
|
Mobile phase A: |
0.1% HCOOH in water |
|
Mobile phase B: |
0.1% HCOOH in acetonitrile |
|
Gradient: |
Start at 15% B and hold for 0.2 min, linear ramp to 50% B at 3.8 min, flush column at 98% B for 0.6 min and return to initial |
|
Flow rate: |
0.4 mL/min |
|
Column temp.: |
60 °C |
|
Sample temp.: |
15 °C |
|
Injection volume: |
30 μL |
|
Run time: |
5.5 min |
|
Collection plates: |
Waters 1-mL ACQUITY collection plates |
|
Mass spectrometer: |
Xevo TQ-S |
|
Ionization mode: |
ESI positive |
|
Capillary voltage: |
3.0 kV |
|
Desolvation temp.: |
600 °C |
|
Cone gas flow: |
150 L/h |
|
Desolvation gas flow: |
1000 L/h |
|
Collision cell pressure: |
3.8 x 10 (-3) mbar |
|
Collision energy: |
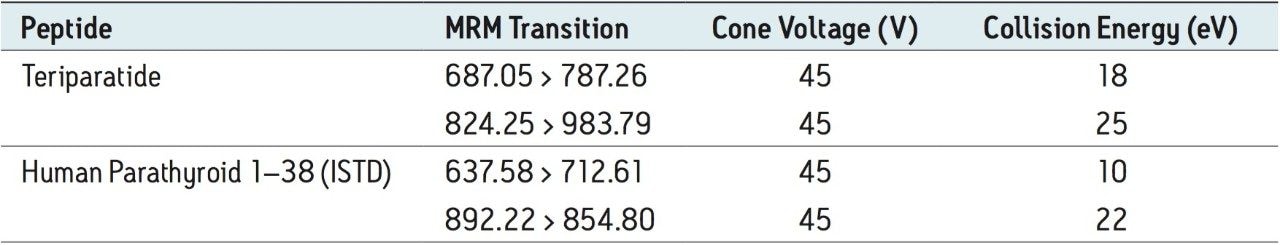
Optimized by component, see Table 1 |
|
Cone voltage: |
Optimized by component, see Table 1 |
|
Data management |
|
|
Chromatography software: |
MassLynx |
|
Quantification software: |
TargetLynx |
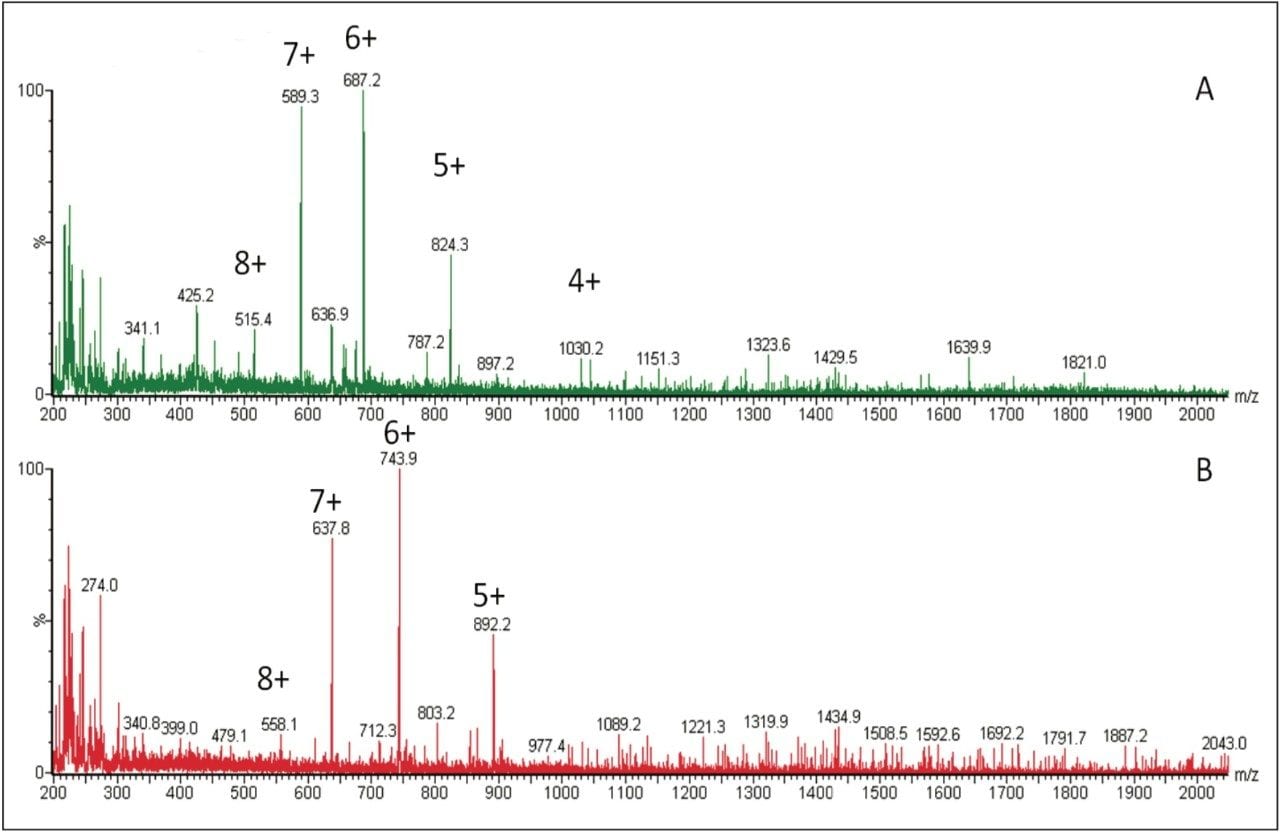
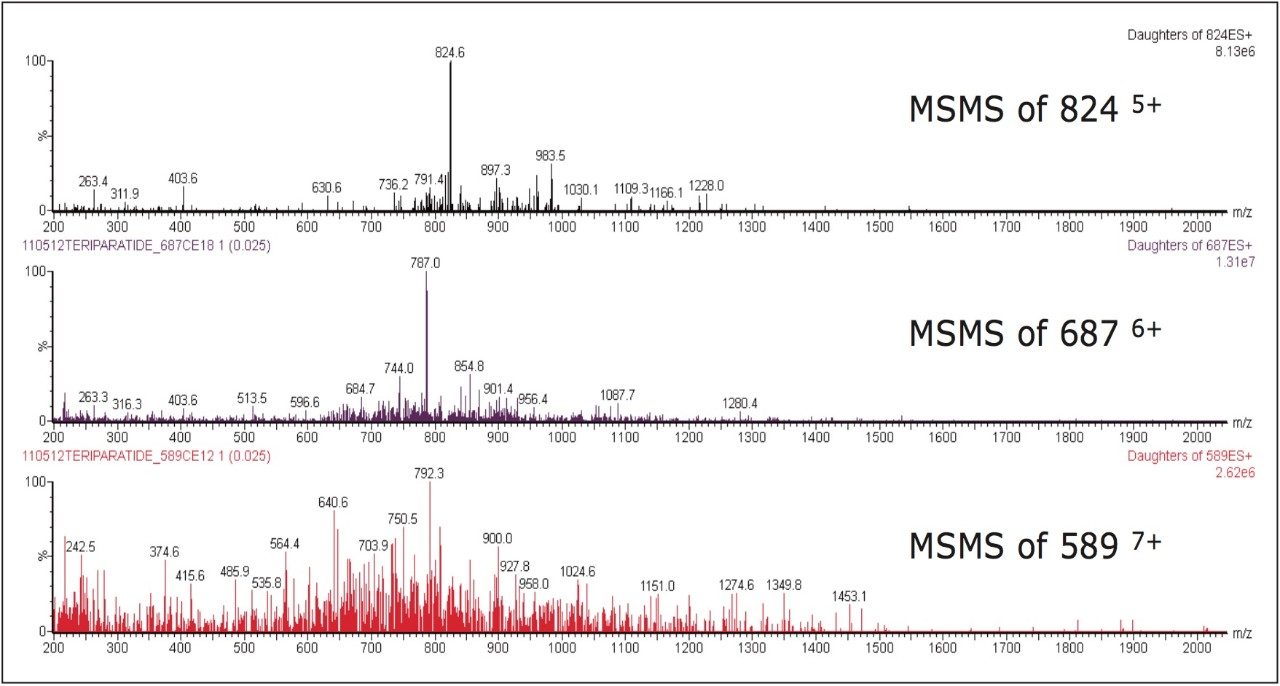
Several multiple-charged precursors were observed for teriparatide and human parathyroid hormone 1–38 (ISTD), with spectra shown in Figure 2. MS/MS spectra for the three most abundant precursors for teriparatide, obtained at their optimal collision energies, are shown in Figure 3. The 6+ charge state of teriparatide at m/z 687 was determined to be the most intense and yielded a selective fragment at m/z 787 for quantitative analysis. The 7+ precursor at m/z 589 was also intense, but did not yield any usable fragments. CID of the 5+ precursor at m/z 824 produced fragment ions of sufficient intensity to be used for confirmatory purposes. Although many peptides produce intense fragments below m/z 200, these ions (often immonium ions) result in high background in extracted samples due to their lack of specificity. In this assay, the use of highly specific y ion fragments above m/z 700 yielded significantly improved specificity, facilitating the use of simple LC and SPE methodologies.
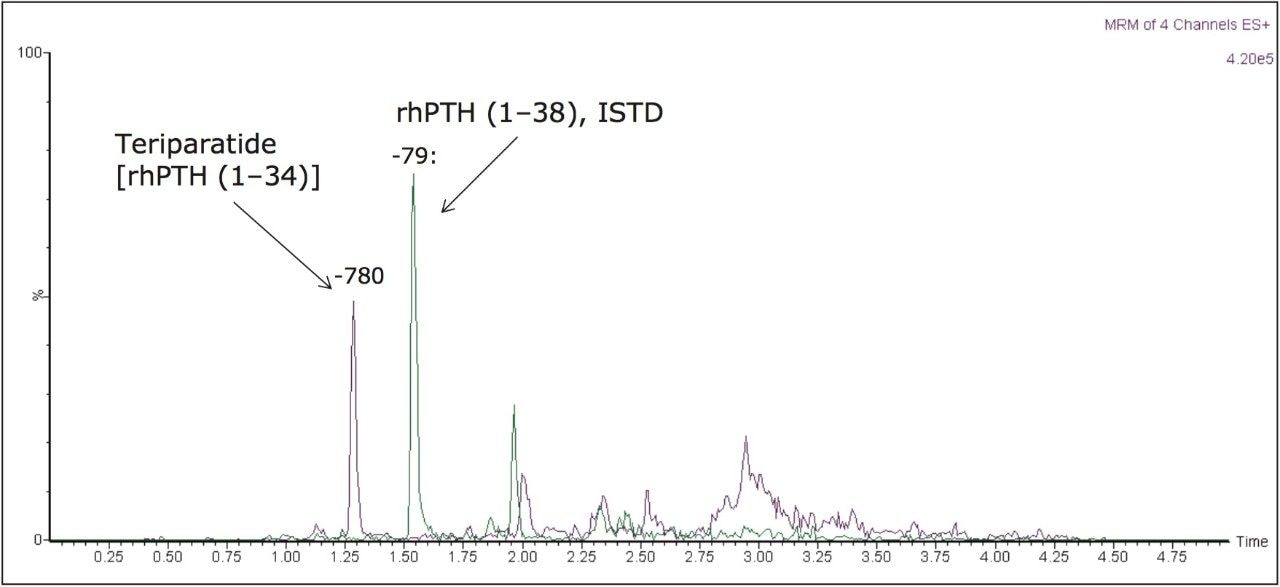
Narrow peak widths were obtained for teriparatide and the ISTD, using a novel charged surface hybrid (CSH) column. The resulting separation of a 125 pg/mL QC sample is shown in Figure 4. Peak widths at base are <6 s wide.
Development of this assay was challenging due to a high degree of non-specific binding (NSB) and difficulty maintaining peptide solubility throughout the SPE extraction and elution processes. Sample pre-treatment prior to SPE proved to be critical to improving recovery and specificity. Protein precipitation (1:1) with 5% NH4OH acetonitrile resulted in 80% to 100% recovery without precipitating the peptide itself. Protein precipitation with higher organic ratios resulted in peptide loss due to undesirable precipitation of teriparatide. The PPT pre-treatment minimized protein binding and eliminated endogenous interferences from large proteins, such as albumin. The supernatant was then applied to conditioned SPE plates, and analytes were well retained on the SPE sorbent during the basic pH load step, with no breakthrough occurring. Optimization of the elution solution was critical to fully elute teriparatide, maintain its solubility, and minimize interferences from the plasma matrix. The optimum elution solution was 60% organic, with 1% trifluoroacetic acid and 5% trifluoroethanol (TFE), the latter being added to maintain solubility of the compound. The addition of TFE also aided in sensitivity and increased the MS signal by 50%. The combination of proper MS fragment choice, selective SPE cleanup, and optimal LC column enabled detection and quantification limits of 15 and 30 pg/mL, respectively, in all six lots of control plasma tested.
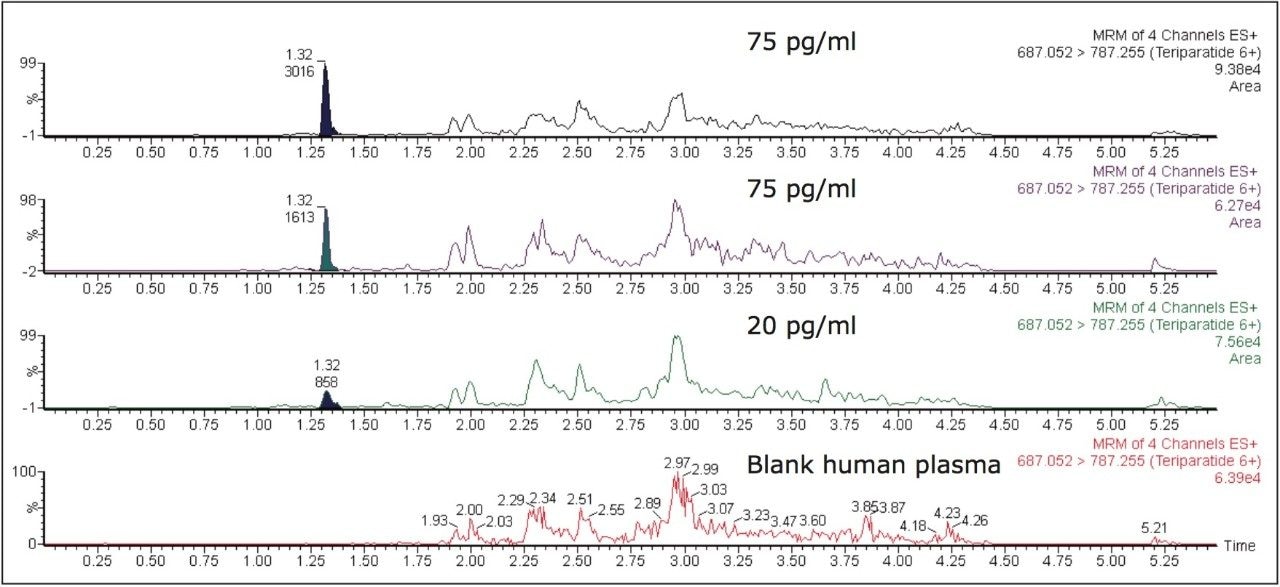
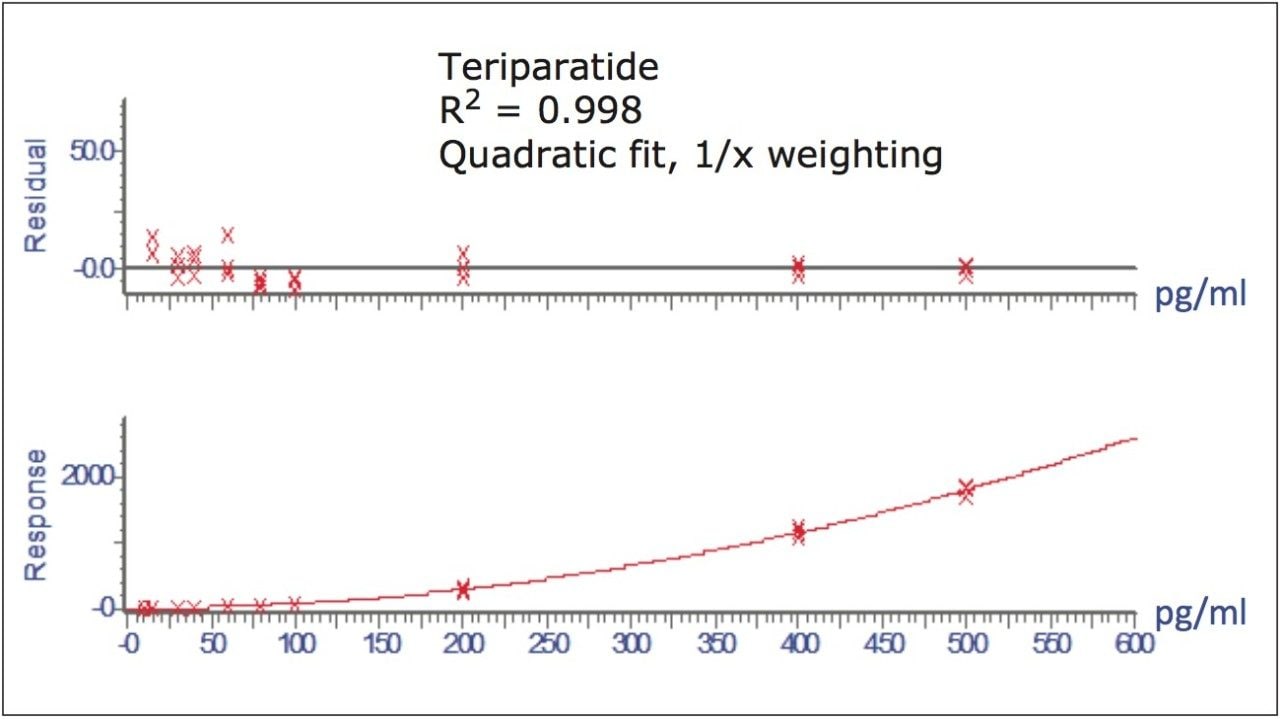
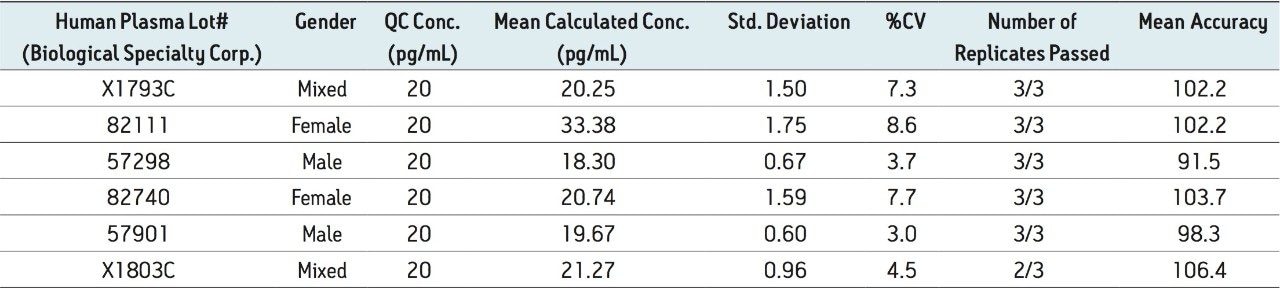
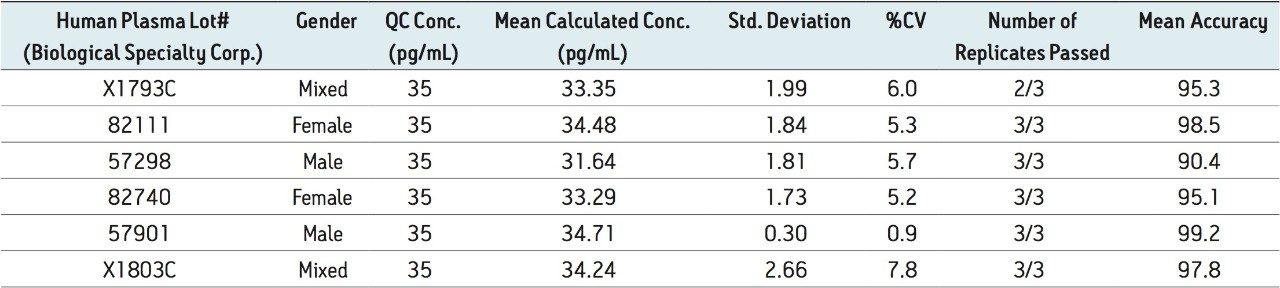
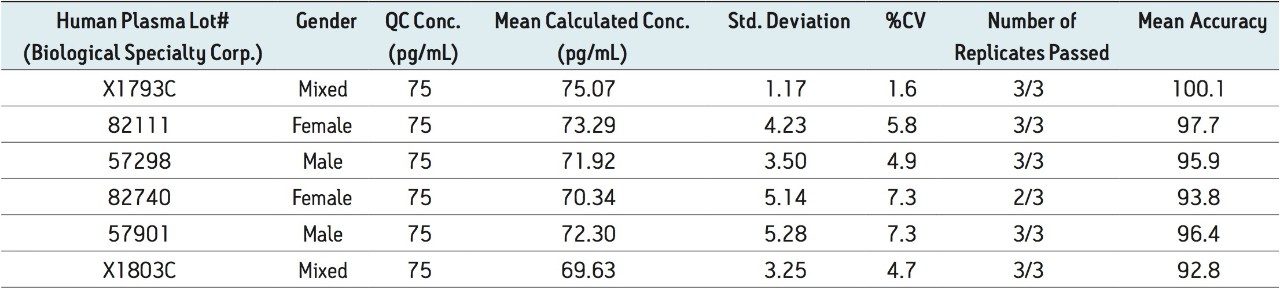
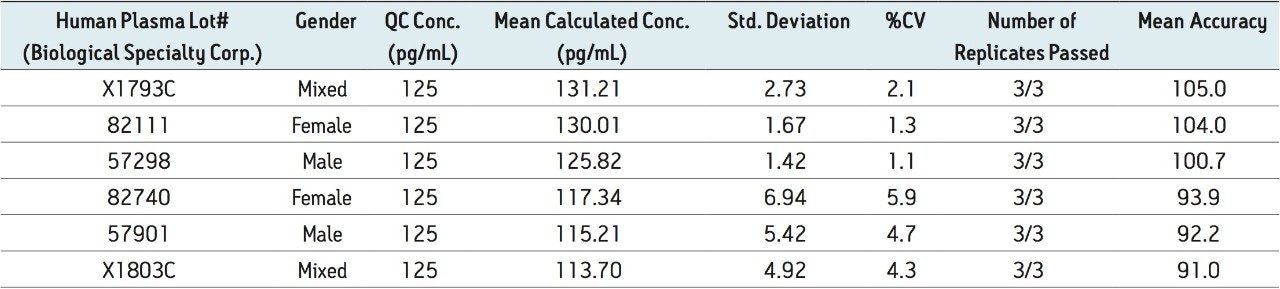
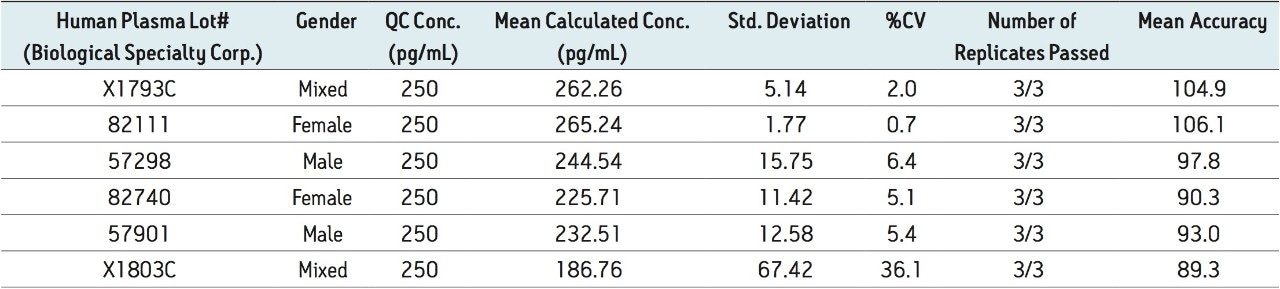
Figure 5 contains a representative chromatogram for low level QC’s samples containing teriparatide at 20, 35, and 75 pg/ml extracted from human plasma as compared to blank extracted plasma. Figure 6 is a representative extracted standard curve for teriparatide, from 15 to 500 pg/mL, in human plasma. Finally, the standard curve and QC statistics for teriparatide are summarized in Tables 2 and 3.
720004588, February 2013