This application note demonstrates the powerful utility of electron transfer dissociation (ETD) and ion mobility mass spectrometry (IM-MS) for structural characterization, by the separation of overlapping isobaric product ions produced from the reducing and non-reducing ends of the oligosaccharides.
The implementation of electron transfer dissociation (ETD) on the SYNAPT G2 High Defintion MS (HDMS) System, a T-Wave ion mobility enabled mass spectrometer, allows two isomeric milk oligosaccharides, LNT and LNnT, to be distinguished from one another. The combination of ETD with ion mobility enables the separation of isobaric product ions produced from the non-reducing and reducing termini of the oligosaccharides.
Glycosylation is the co- and post-translational modification of carbohydrate structures to a protein backbone, and represents ~ 50% of all proteins in eukaryotic systems. The carbohydrate moieties play important roles in biological systems, such as folding, energy generation, stability, and cell-cell interactions. Changes in the glycosylation profiles of specific proteins have been recognized as disease markers. More than one-third of approved biopharmaceuticals are glycoproteins.
Glycoproteins can be either N-linked or O-linked. In N-linked glycosylation, oligosaccharides bind to the side chain nitrogen of asparagine where the asparagine forms part of an Asn-X-Ser/Thr consensus sequence where X can be any amino acid except proline. O-linked glycans bind to the hydroxyl oxygen of serine and threonine side chains, and do not have a consensus sequence. Addition of carbohydrate-containing glycosylphosphatidylinositol anchors to proteins, allowing for membrane attachment, can also be considered a form of glycosylation.
Despite the importance of oligosaccharides in biological systems, structural determination of these molecules is analytically challenging compared to other biomolecules. Carbohydrates present a wide structural diversity because of variability in interglycosidic linkages and branching, even with a very limited set of monosaccharides. Given that monosaccharides have multiple linkage sites and each site has two possible anomeric linkage configurations, the structural characterization of oligosaccharide structures can be very complex.
In this study, two isomeric milk oligosaccharides, Lacto-N-tetraose (LNT, Galβ1→3GlcNAcβ1→3Galβ1→4Glc) and Lacto-N-neotetraose (LNnT, Galβ1→4GlcNAcβ1→3Galβ1→4Glc) were investigated using ETD-ion mobility mass spectrometry (IM-MS). The practical application of ETD combined with ion mobility is demonstrated for the separation of isobaric product ions.
The LNT and LNnT samples supplied for analysis underwent a derivatization step by permethylation. The two samples were dissolved to a concentration of 3 µM (50% aqueous methanol). Magnesium chloride was used as the cationising reagent and prepared to a concentration of 300 µM (50% aqueous methanol). For ESI-MS analysis, the individual oligosaccharides were mixed with an equal volume of the salt solution.
|
Mass spectrometer: |
SYNAPT G2 HDMS |
|
Ionization mode: |
ESI positive |
|
Capillary voltage: |
3.0 kV |
|
Cone voltage: |
25 V |
|
Desolvation temp.: |
200 °C |
|
Source temp.: |
110 °C |
|
Ionization mode: |
Glow discharge negative |
|
Glow discharge: |
100 μA |
|
Trap T-Wave velocity: |
300 m/s |
|
Trap T-Wave amplitude: |
0.2 V |
|
T-Wave ion mobility cell: |
N2 at 3.5 mbar |
|
T-Wave velocity: |
650 m/s |
|
T-Wave amplitude: |
25 V |
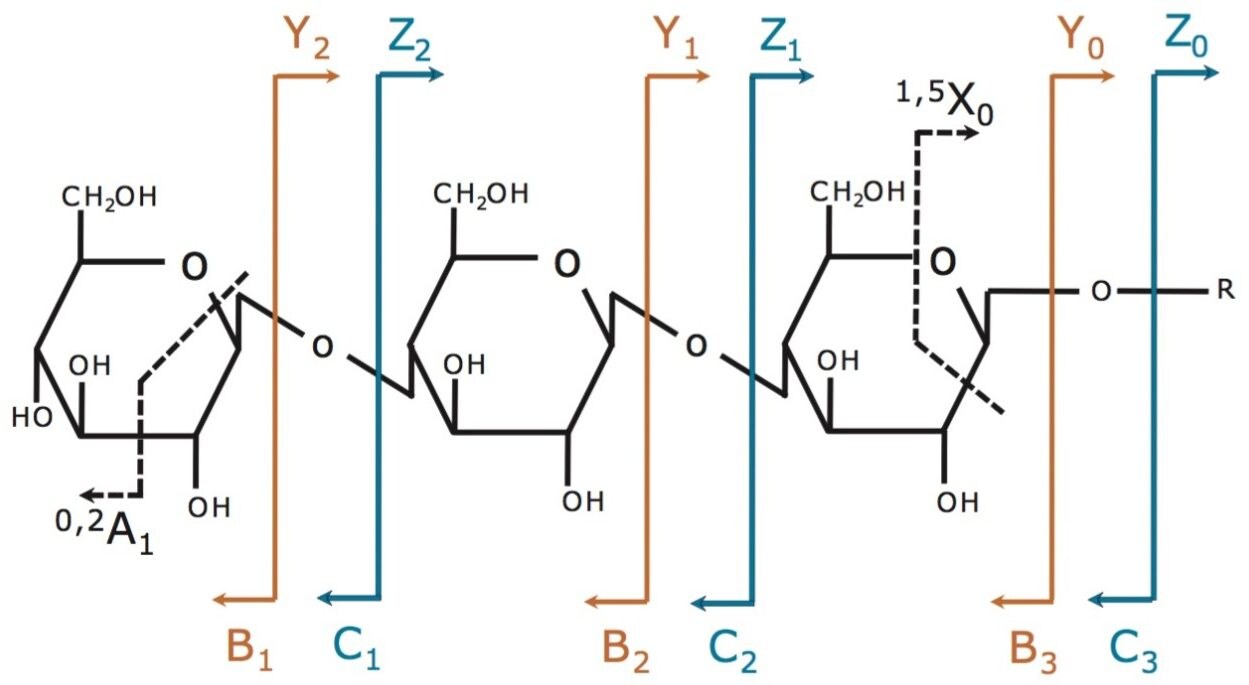
The fragmentation annotation is shown in Figure 1. Product ions that contain a non-reducing terminus are labeled with the uppercase letters A, B, and C. Product ions that contain the reducing end are labeled with letters X, Y, and Z. Subscripts indicate those cleaved ions. The A and X ions are produced by cleavage across the glycosidic ring, and are labeled by assigning each ring bond a number (counting clockwise from the ring oxygen).
Derivatization by permethylation is an important step for the structural characterization of oligosaccharides using mass spectrometry-based methods. As a consequence, hydrogen atoms bound to oxygen and nitrogen atoms are replaced by methyl groups. Permethylated oligosaccharides are more stable than native glycans, and the fragmentation data yielded from tandem mass spectrometry experiments is structurally more informative. During this analysis, the permethylated oligosaccharides were cationised with the divalent metal ion magnesium, leading to the formation of doubly charged precursor ions of the form [M+Mg]2+.
It has recently been demonstrated that the dominant fragmentation pathways following ETD of permethylated oligosaccharides were cross-ring cleavages, in contrast to the much more prominent glycosidic cleavages obtained using CID.2 Figures 2 and 3 show the ETD spectra generated for LNT and LNnT, respectively. The spectra are similar; however, the ion detected at m/z 228 in the ETD spectrum for LNT, labeled E2, an internal ion formed via two glycosidic cleavages was more abundant than for LNnT.
![ETD mass spectrum of [M+Mg]2+, m/z 463.7 from LNT.](/content/dam/waters/en/app-notes/2013/720004476/720004476en-f2.jpg.82.12-16-1268-870C.resize/img.jpg)
![ETD mass spectrum of [M+Mg]2+, m/z 463.7 from LNnT.](/content/dam/waters/en/app-notes/2013/720004476/720004476en-f3.jpg.82.11-19-1264-865C.resize/img.jpg)
The CID spectra of the two permethylated compounds show differences because of the loss of methanol from this ion in the spectrum of LnNT, due to the expected elimination of methanol when the methoxy group is adjacent to the N-acetyl group. The presence of this particular ion at m/z 228 and the relative abundances of other fragments can be used to differentiate the two isomeric permethylated oligosaccharides due to the differences in linkage position, as previously discussed.2 Figure 4 shows the ETD product ion assignments for LNT and LNnT, respectively.
![ETD product ions of [M+Mg]2+, m/z 463.7 from LNT (A) and LNnT (B).](/content/dam/waters/en/app-notes/2013/720004476/720004476en-f4.jpg.82.13-20-937-1181C.resize/img.jpg)
Following ETD fragmentation of the precursor ion [M+Mg]2+, m/z 463.7 from both LNT and LNnT, two abundant singly charged product ions, corresponding to glycosidic cleavage of the GlcNAc-Gal bond were observed. These ions, labeled Y2 and B2 (H), were detected at m/z 463.2 and m/z 464.2, respectively, as shown in the bottom spectrum of Figure 5. It can be seen that the isotopes of the precursor ion together with the two product ions show interference and clearly overlap.
Ion mobility separation of the ETD product ions has the potential to simplify the resultant ETD mass spectra. It also aids detection and interpretation. When these overlapping ions have different mobilities, they can be temporally separated and independently detected. Even if m/z interferences are not present, mass spectral complexity can be high and software-based removal of mobility separated background ions (for example, based on charge state) can simplify resultant mass spectra and aid detection. To illustrate the benefit of ETD combined with ion mobility, example data are shown in Figure 5 for LNT.
![Part ETD-spectrum of [M+Mg]2+, m/z 463.75 from LNT highlighting overlapping ions (bottom spectrum) as observed without ion mobility.](/content/dam/waters/en/app-notes/2013/720004476/720004476en-f5.jpg.82.11-28-1233-1062C.resize/img.jpg)
The inset shows the arrival time distribution (ATD) for the product ion B2(H) centered around 3.19 ms and that of the precursor ion [M+Mg]2+ centered around 2.01 ms, appearing at the bottom. Since these ions have different charges, they have significantly different ion mobilities and can be independently detected. The monoisotopic mass of Y2 does not have any interferences, and has an ATD centered around 3.05 ms, as seen at the top. By combining the mass spectra over the individual peaks in the ATD, mass spectra were independently obtained. This is shown for the Y2 (middle spectrum) and B2(H) (top spectrum). The ion mobility data suggests that although the B2(H) and Y2 product ions are of similar m/z, the structure of the Y2 ion is more compact than the B2(H) ion based on the difference in the ATD.
We would like to thank Professor Catherine Costello, Boston University School of Medicine, for the generous gift of the samples used in this study.
720004476, January 2013