For research use only. Not for use in diagnostic procedures.
ETD is a powerful fragmentation technique that has proved particularly useful for the determination of labile post-translational modifications (PTMs) of peptides and proteins. In this application note, we demonstrate the advantages of Electron Transfer Dissociation (ETD) for the for site-specific peptide octanoylation in Human Ghrelin.
The technique has been used to determine the site-specific octanoylation of the peptide Ghrelin which could not be determined using CID.
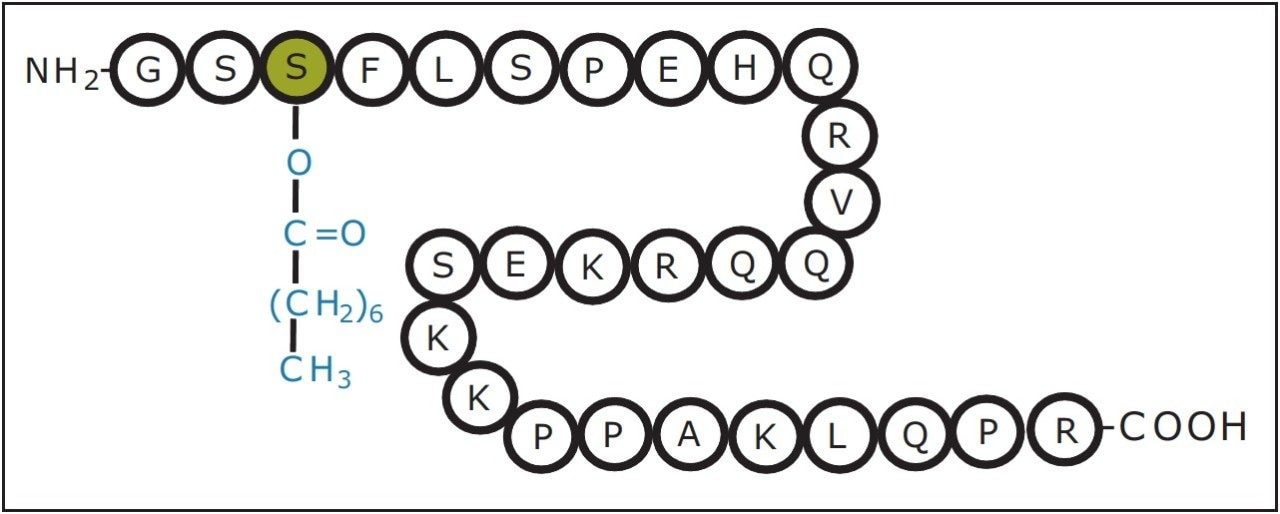
Ghrelin (MWmonoisotopic = 3368.9, C149H249N47O42) is a human peptide hormone containing 28 amino acid residues, which is primarily produced in the stomach. The biological function of Ghrelin is known to stimulate the brain to increase appetite and favor the accumulation of lipids in visceral fatty tissue. The amino acid residue at the third position (Ser3) needs to be octanoylated (modified with a moiety of 126 Da, C8H14O), in order to become biologically active, as shown in Figure 1. The acylation is necessary for the peptide to bind and activate with its receptor, growth hormone secretagoue receptor (GHS-R).
In this application note, we demonstrate the advantages of Electron Transfer Dissociation (ETD) for the for site-specific peptide octanoylation in Human Ghrelin.
ESI-MS was performed on a hybrid quadrupole/ion mobility/oa-ToF mass spectrometer fitted with electron transfer dissociation (ETD) functionality. In brief, the instrument comprises three consecutive, gas filled, travelling wave (T-Wave) RF stacked ring ion guides prior to the ToF mass analyzer. For ETD type fragmentation, a sub-ambient pressure (~2 mbar) glow discharge anion source was used to fill the Trap T-Wave cell with quadrupole mass selected ETD reagent anions formed from para-nitrotoluene (m/z 137). During an acquisition, the source polarity and quadrupole set mass are switched to allow multiply charged cations formed from ESI of the peptides to interact with stored reagent anions in the Trap T-Wave. This interaction allows an ion-ion type reaction resulting in ETD product ions. For efficient ETD within the Trap T-Wave cell, the bath gas used was helium set to a pressure of 0.05 mbar. The Transfer T-Wave cell was pressurized to 0.005 mbar with argon. The T-Wave speed and amplitude which influence the ion-ion interaction time, as well as the reaction rate were set to 300 m/sec and 0.2 V respectively. Solutions were infused into the source region of the MS at a flow rate of 5 μL/min.
|
MS system: |
SYNAPT G2 |
|
Ionization mode: |
ESI positive |
|
Capillary voltage: |
3.5 kV (for positive) |
|
Cone voltage: |
30 V |
|
Desolvation temp.: |
200 °C |
|
Source temp.: |
120 °C |
|
Ionization mode: |
Glow discharge negative |
|
Glow discharge: |
55 μA (for negative) |
|
Reagent: |
para-nitrotoluene (m/z 137) |
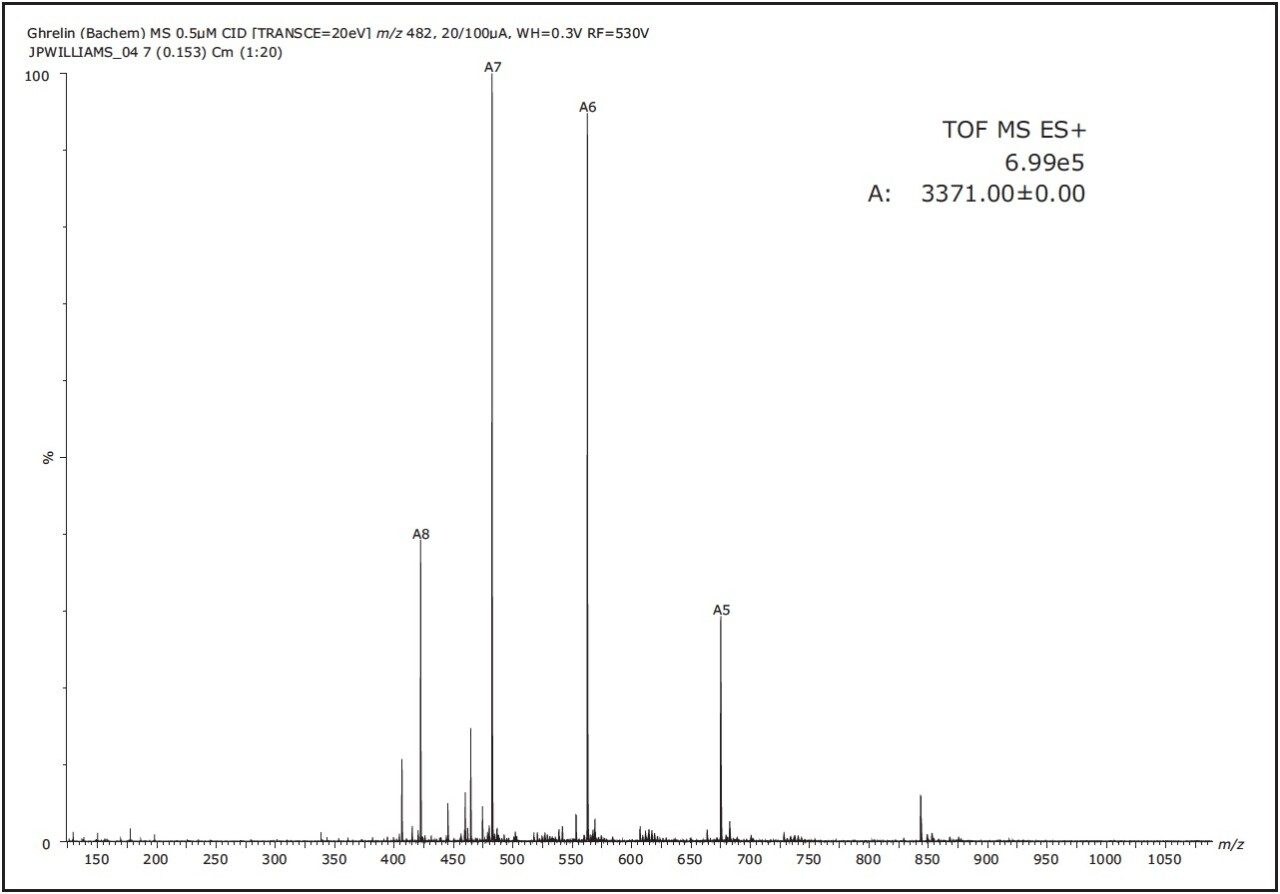
Figure 2 shows the SYNAPT G2 ESI mass spectrum of Ghrelin. Multiply-charged ions were detected from [M+4H]4+ to [M+8H]8+ with the base peak in the spectrum corresponding to [M+7H]7+.
The most abundant multiply charged precursor ion ([M+7H]7+ at m/z 482.3) was selected using the quadrupole to undergo low energy CID in the Transfer Triwave region of the instrument. CID causes vibrational excitation and predominantly breaks backbone amide bonds. Figure 3 shows the deconvoluted MS/MS mass spectrum of the precursor after Maximum Entropy 3 processing. This spectrum was obtained using argon as the collision gas (pressurized to appproximately 5 x 10-3 mbar) and ion collision energy of 15 eV.
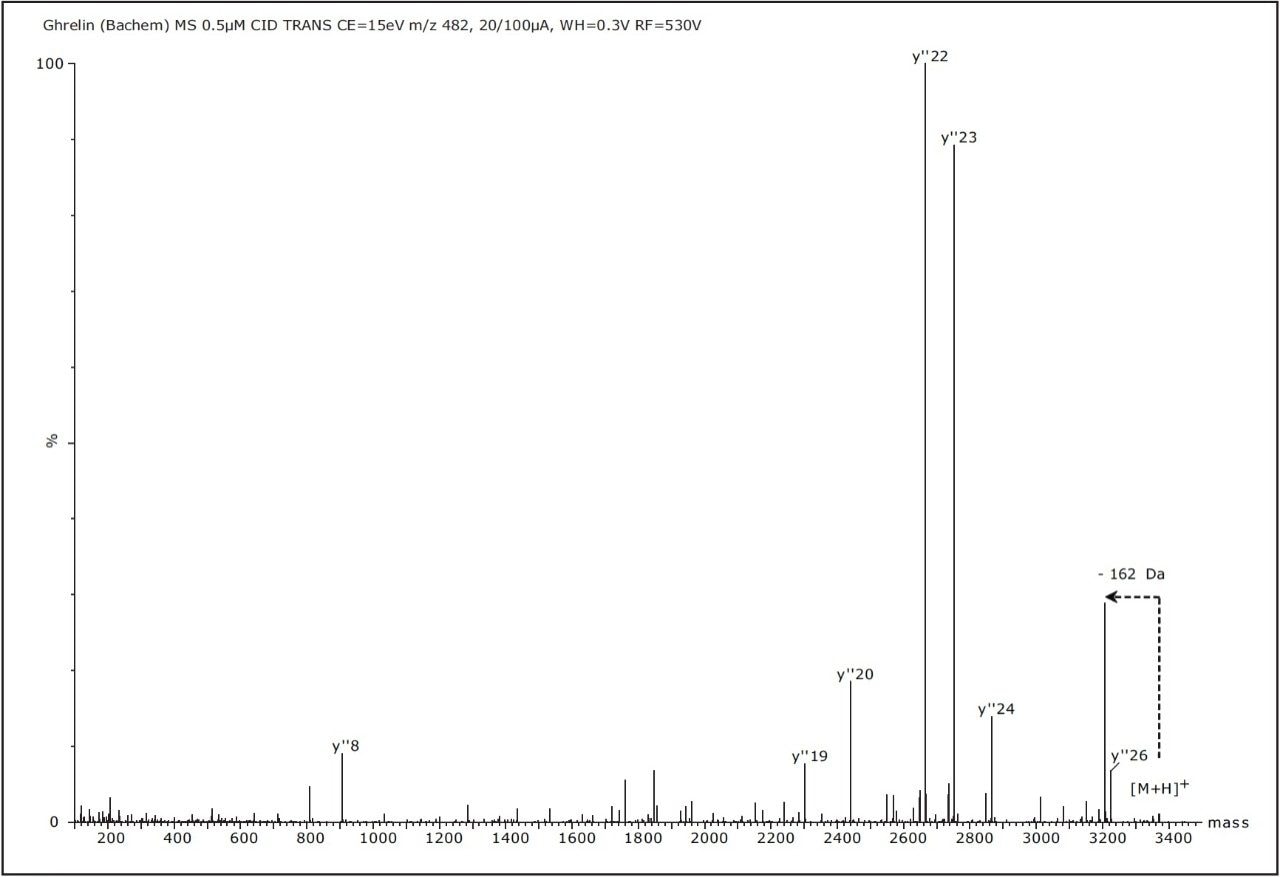
The most abundant product ions detected in Figure 3 correspond to C-terminal y"19-24 with very few N-terminal b ions observed. The only observed ions containing octanoate modification is the y"26 ion. Neutral losses corresponding to octanoic acid and water (162 Da) were also observed in the spectrum.
The CID data show that the MS/MS spectrum yielded an incomplete sequence coverage of Ghrelin, because of the presence of multiple basic amino acid residues (K or R), and four proline residues. This partial sequence coverage, especially the lack of complimentary b and y ions that cover the acylation site, makes the deduction of the octanoylation less convincing. Alternative fragmentation method was tested in the following step.
ETD is a powerful fragmentation technique that has proven particularly useful for the determination of labile post-translational modifications (PTMs) of peptides and proteins. ETD is a radical-driven fragmentation technique and results in cleavage of the peptide N-Cα bond to give c and z• type peptide product ions. On a SYNAPT G2 HDMS System, reagent anions generated using low-pressure glow discharge produced from 4-nitrotoluene (m/z 137) were used for ETD experiment. The nitrogen make-up gas flow (at 20 mL/min) carried the reagent vapor to the tip of the discharge cathode (at 55 μA) for the generation of radical anion. The ion source polarity and the quadrupole set mass were sequentially switched to deliver [M+7H]7+ and singly-charged 4-nitrotoluene radical anions into the Trap T-Wave (pressurized to 5 x 10-2 mbar with helium) cell, where the cations and anions interacted to give ETD type fragmentation. Figure 4 shows the Maximum Entropy 3 deconvoluted ETD mass spectrum of the precursor [M+7H]7+.
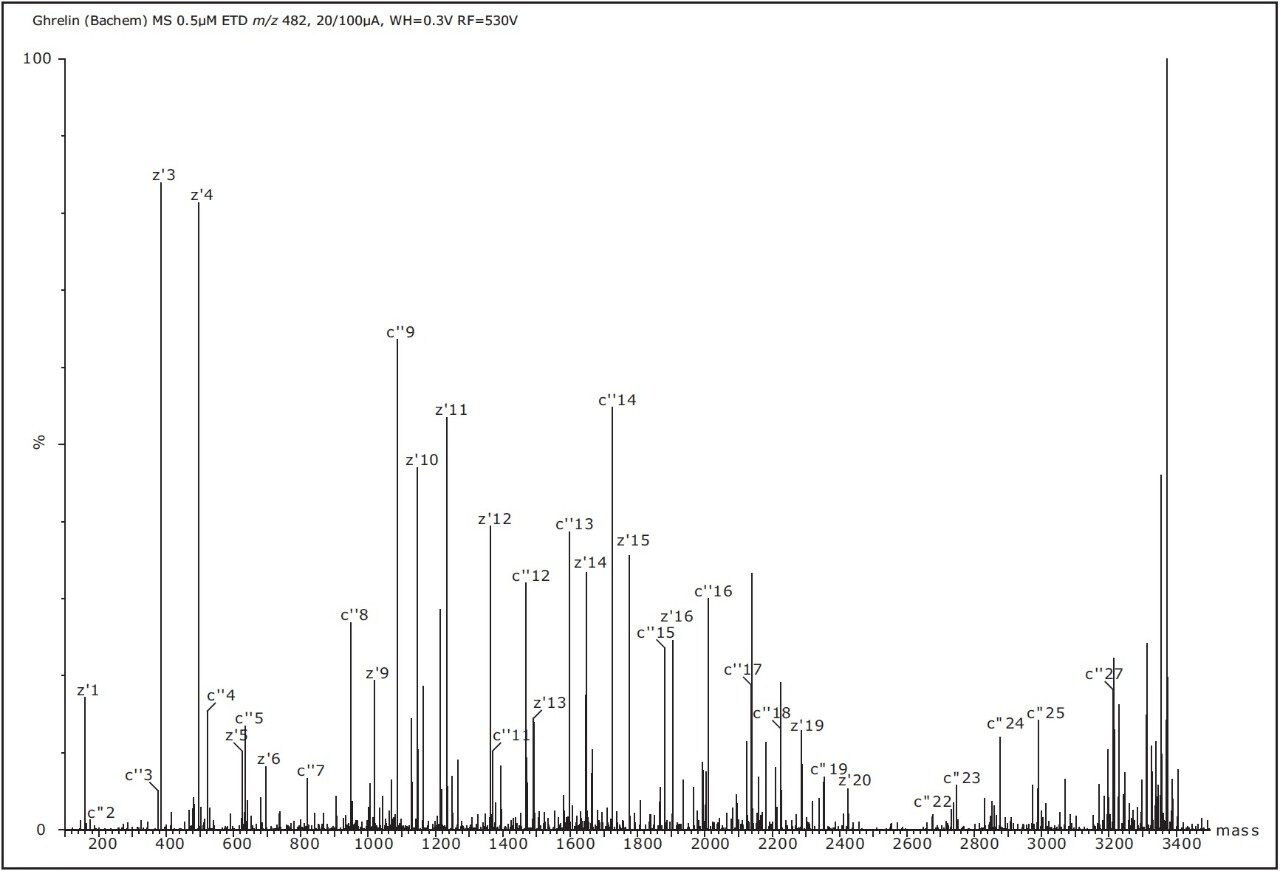
ETD provided excellent sequence coverage compared to CID fragmentation. The peptide is comprised of four proline (Pro) residues, and because of the cyclic structure of proline, N-terminal dissociation adjacent to this residue is typically not observed using ETD. Instead, N-terminal, c ions (even-electron) were detected for c"2 +-c"5 +, c"7 +-c"19 +, c"22 +-c"25 + and c"27 +. Odd-electron, C-terminal z ions were detected for z'1 +•- z'6 +• and z'9 +•- z'21 +• and z'26 +•- z'27 +•. Thus, the mass difference of 213 Da between c"2 + and c"3 + clearly shows the mass of the octanosylated serine residue modified with C8H14O. These ions were not observed in a previous study using ECD in a FTICR-MS type instrument.1
720004290, April 2012