For research use only. Not for use in diagnostic procedures.
This is an Application Brief and does not contain a detailed Experimental section.
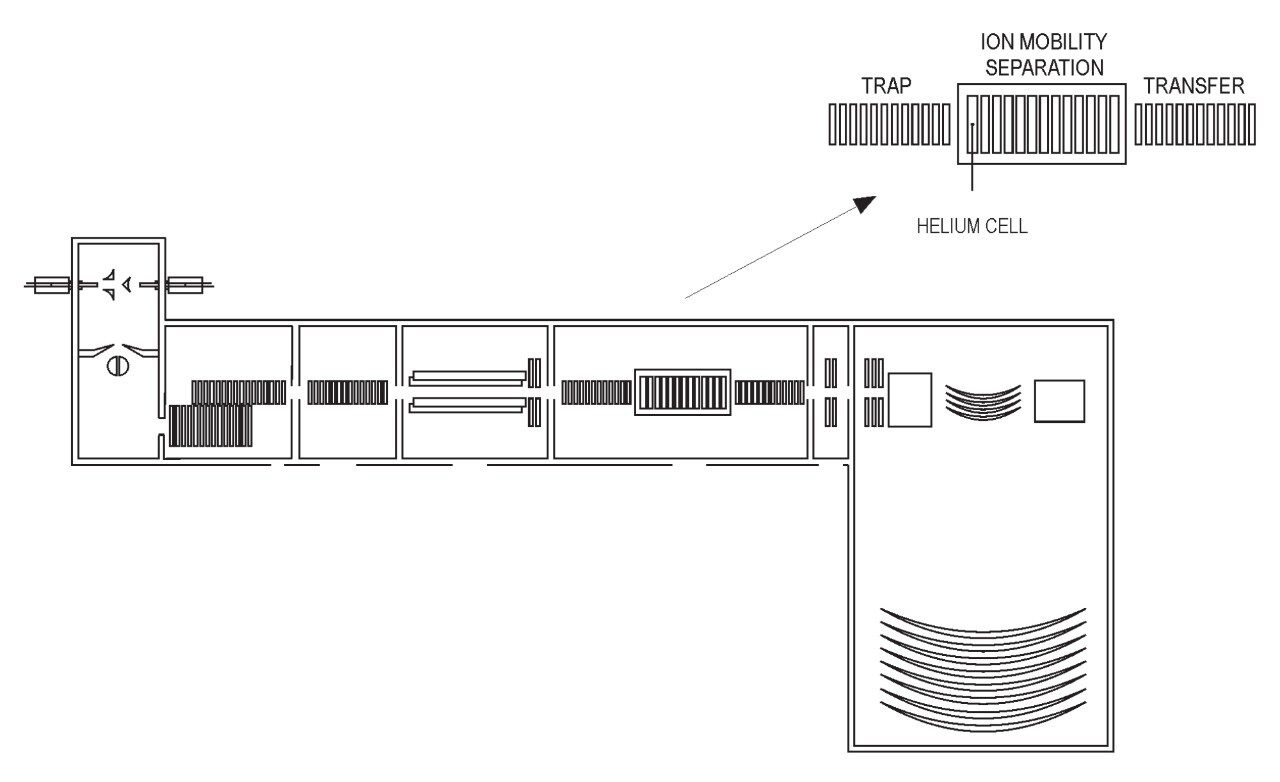
This application brief evaluates the performance of SYNAPT G2-S incorporating StepWave Technology for the rapid analysis of intact proteins using Electron Transfer Dissociation (ETD).
Rapid top-down ETD analysis using travelling wave ion guide technology.
ETD is a fragmentation method that can be applied for the structural characterization and subsequent identification of intact proteins. This so-called ‘top-down’ proteomics approach is fast, because no enzymatic digestion step is necessary, and the method has been shown to require only low attomole to femtomole amounts of sample.
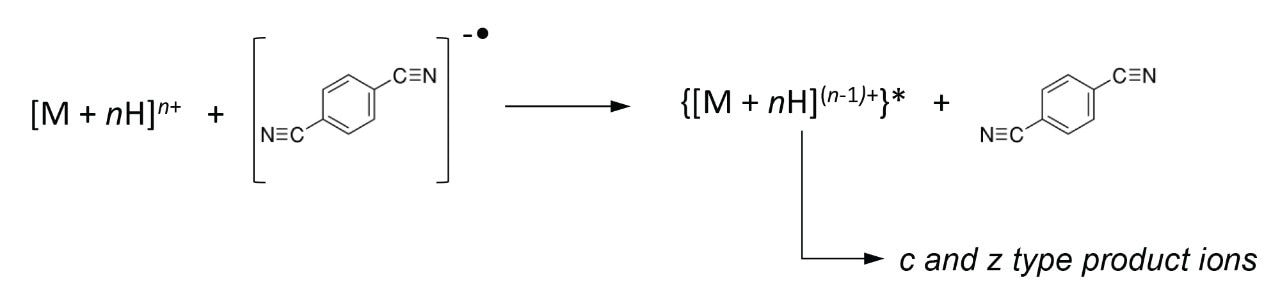
The ETD method is known to provide vast N-C backbone cleavage (c and z ions), preserves weakly bonded post-translational modifications, and it has the potential for cleaving disulphide bonds. Taken together, the attributes of the ETD technique combined with the enhanced sensitivity and speed of Waters SYNAPT G2-S may offer a suitable platform for intact protein analysis, particularly hydrophobic proteins, compared to the more routinely used ‘bottom-up’ approach for protein identification.
Bovine Carbonic Anhydrase II (BCA) was dissolved to a concentration of 100 fmol/ L (50% aqueous acetonitrile containing 0.2% formic acid). The solution was infused into the source region of the instrument at a flow rate of 4 L/min. The total acquisition time was 1 minute. Tandem mass spectra were recorded on an ETD enabled SYNAPT G2-S Mass Spectrometer. Gas phase ion-ion chemistry for protein fragmentation using ETD was induced by selecting both BCA precursor cations retaining 35 charges (m/z 830), and precursor anions formed from 1,4-dicyanobenzene (m/z 128) using the quadrupole analyzer (1 s polarity switch during acquisition). Following quadrupole selection, shown in Figure 1, these ions interacted within the trap travelling wave ion guide of the instrument. The ETD fragmentation process is illustrated in Figure 2 for peptide and protein type analysis. Figure 3 shows the analysis of intact BCA using ETD. The spectrum is relatively complex and interpretation was simplified through the use of BioLynx Software for dedicated ETD product ion annotation. This short acquisition allowed for the annotation of approximately 40 N-terminal (c ions) and 40 C-terminal (z ions) product ions following ETD.
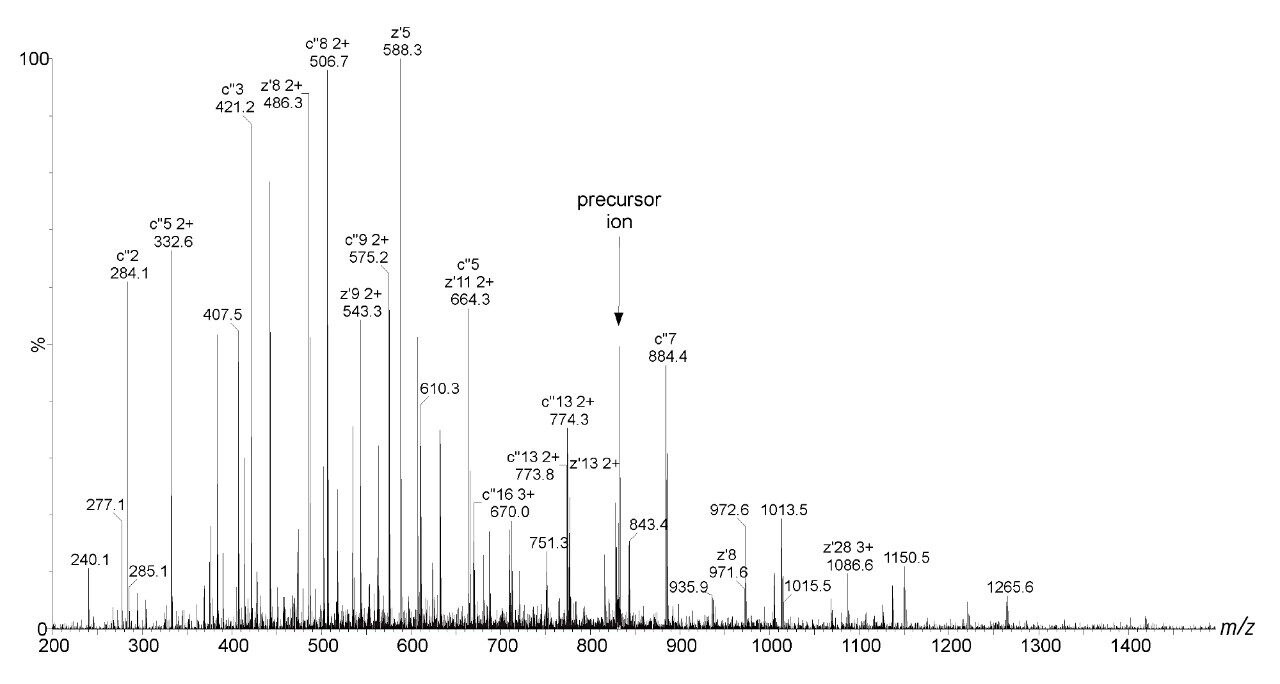
This application brief shows that the implementation of ETD on the SYNAPT G2-S provides a beneficial means for analyzing intact proteins using a ‘top-down’ approach. ETD affords extensive N-C backbone cleavage for sufficient structural characterization and subsequent identification of intact BCA on a rapid timescale.
720004154, December 2011