This is an Application Brief and does not contain a detailed Experimental section.
This Application brief demonstrates reliability of the quaternary-based ACQUITY UPLC H-Class Bio System and the ACQUITY UPLC BEH200 SEC Column for the analysis of proteins by size exclusion chromatography (SEC).
The ACQUITY UPLC H-Class Bio System along with an ACQUITY UPLC BEH200 SEC Column deliver reliable and reproducible SEC separations for biomolecules.
The complete characterization and analysis of biopharmaceuticals includes the application of size exclusion chromatography (SEC) to measure protein aggregates and other size variants. Soluble protein aggregates, in particular, can contribute to immunogenicity; accurate analysis and quantitation of biotherapeutic protein aggregates is, therefore, often required.
Current HPLC/silica-based SEC methods can be time-consuming and unreliable. These uncertain results may be due to changes in retention time, peak shape, or spacing between peaks as well as irreproducibility between columns and changes in columns within a few runs.
With the introduction of the ACQUITY UPLC H-Class Bio System and sub-2-μm ACQUITY UPLC BEH200 SEC Column chemistry, SEC separations can be obtained reproducibly, reliably, and in shorter analysis time with minimal development. Methods can be easily developed with the system’s quaternary solvent manager utilizing Auto•Blend Plus Technology. This new implementation of instrument control functions removes the need for buffer pH adjustment and reduces time spent in buffer preparation.
The superior performance of this UPLC SEC method relies on both the inert, low-dispersion system and the chemically-stable BEH column. The combination of these components allows users to obtain more accurate and reproducible results over a larger number of samples than is observed with current SEC methodologies.
The SEC separation of biomolecules combines the ACQUITY UPLC H-Class Bio System with a 1.7-μm ACQUITY UPLC BEH SEC Column that provides the biochemist with a reliable separation. The lowdispersion, high-pressure system contains an inert flow path, that, when combined with four-solvent mixing and Auto•Blend Plus Technology, facilitates easy buffer preparation without pH adjustment.
The ACQUITY UPLC BEH200 SEC particle has an effective diol coating that provides a stable particle with minimal secondary interactions. The packing material is more resistant to chemical and mechanical degradation over time. These attributes combine to provide an SEC column stable more than 600 injections and requiring lower buffer concentrations than traditional silica-based columns.
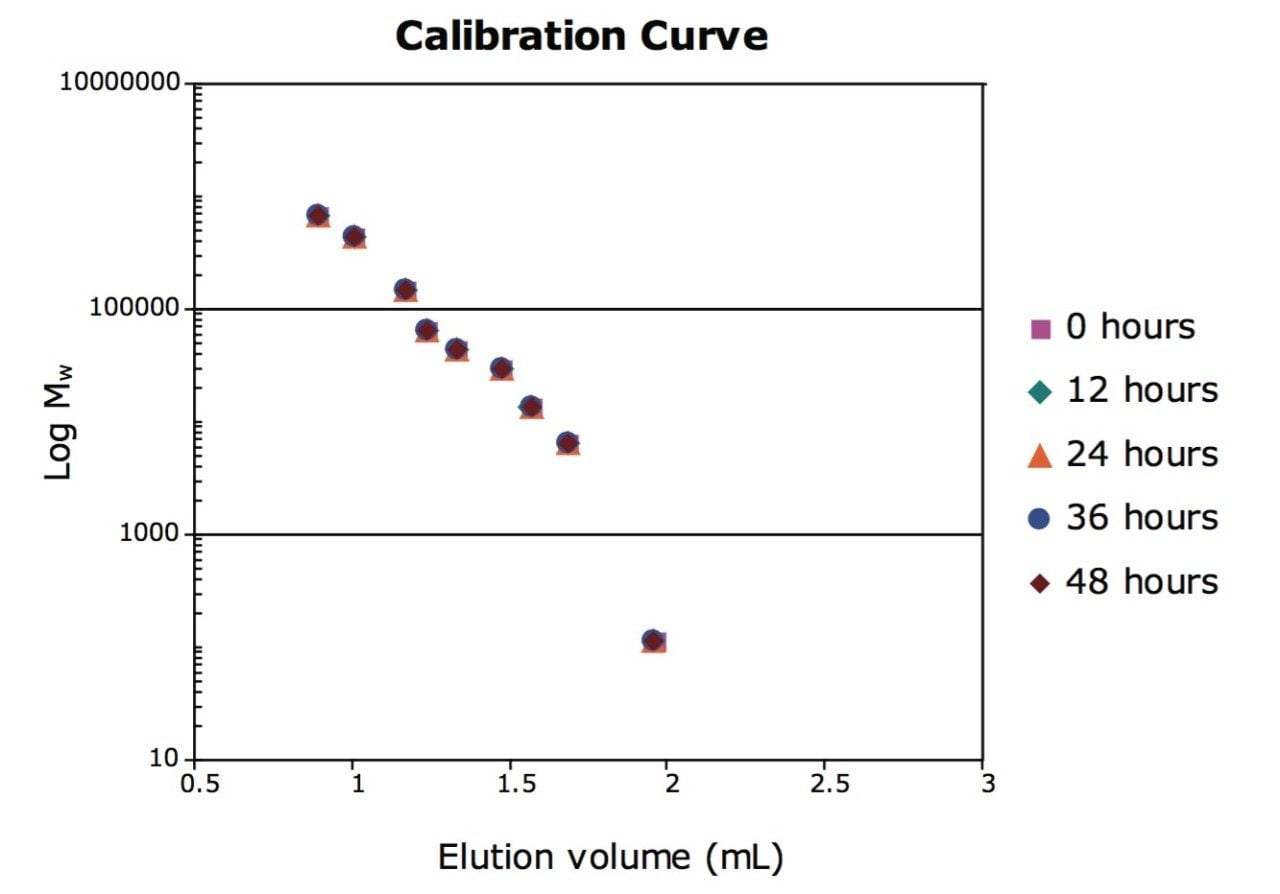
In a series of experiments, protein standards and monoclonal antibody biotherapeutics were analyzed with UPLC-based SEC. Repeated analysis of the same sample was performed at regular intervals over a two-day period. Reproducibility of the calibration was tested by analysis of proteins standards over the molecular weight range of 10,000 to 450,000 Da.
The elution volume for each protein standard was found to be within 0.2% RSD. The calibration curve points do not fall on a perfect straight line because the elution volume reflects both size and shape of protein standard. The consistency of the calibration curve is, however, indicative of both the column life and instrument control of flow rate and injection volume.
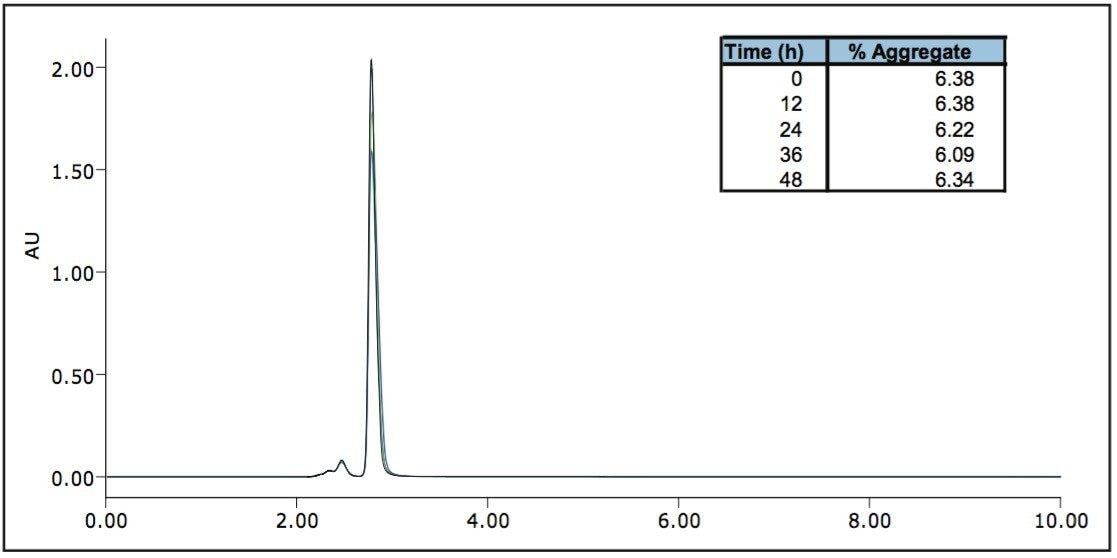
To test the reliability of quantitation, a humanized monoclonal antibody was analyzed. The sample shown was found to have an average aggregate quantitation of 6.82% +/- 0.3% of the monomeric species over the time period. The reliability of this analysis is demonstrated by the reproducibility of this measurement. The SEC separations demonstrate the accuracy and reproducibility of UPLC SEC technology, which, in turn, ensures accurate identification and aggregate determination.
720003602, June 2010