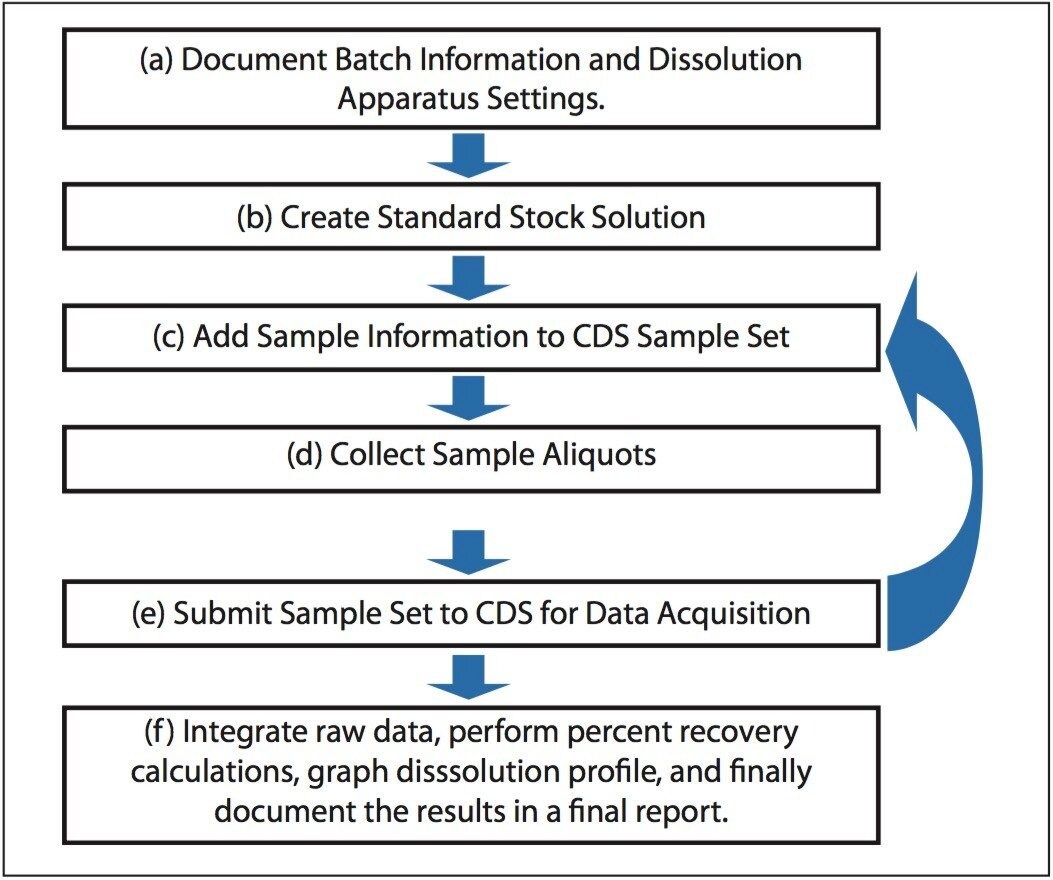
Dissolution studies during drug product manufacturing employ a standardized workflow that may be readily captured as a standardized template by using electronic report and authoring tools. A standardized template both guides the analyst through the workflow and ensures reliable data capture without skipping necessary steps.4
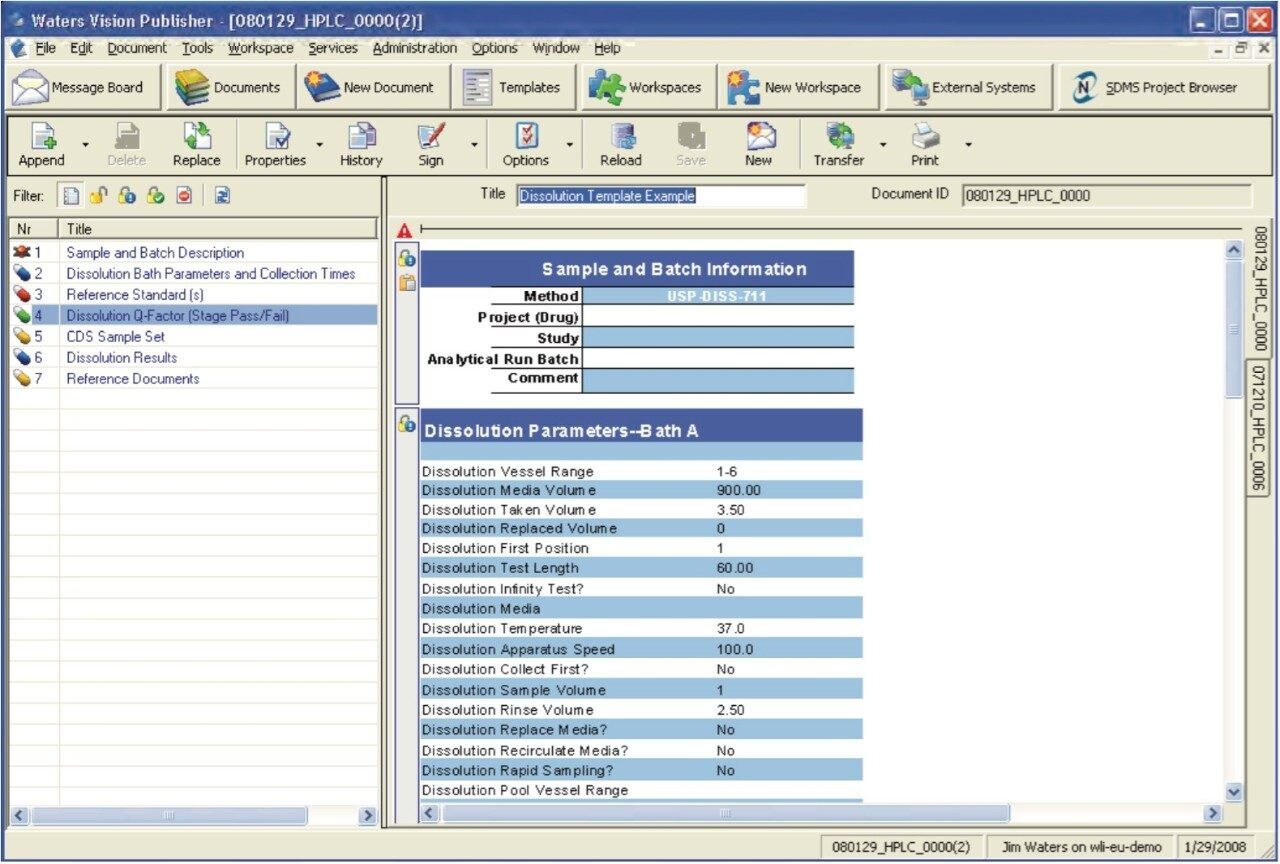
SDMS Vision Publisher is a reporting and authoring module for NuGenesis SDMS. A Vision Publisher Dissolution Template provides a dissolution workflow and acts as a centralized document to record all aspects of a dissolution study.
In vitro dissolution simulates in vivo drug release of solid dosage forms, transdermal patches, and suspensions.1-3 For example, in the case of tablets and capsules, the acidic environment of the stomach is emulated and the rate of drug release is measured as a function of time. Quantitative measures are made by taking small aliquots of the dissolution medium from the dissolution device at defined time points, e.g., 5, 10, 20, 30 minutes.1-3 The measurements can be performed by a number of standard analytical instruments with UV/Vis spectrometers and HPLC being some of the most common.1 In particular, HPLC methodology offers the advantages of wide dynamic range and excellent selectivity because of the chromatographic separation aspect.1
Dissolution testing may be performed during various phases of the drug development lifecycle of a dosage form, e.g., during Phases 0 and I to understand the dissolution mechanism; during Phases II and III to understand formulation; and as quality control (QC) during manufacturing. 1-2 Maintaining good documentation of the dissolution studies is a common theme no matter what the stage of the product life-cycle but is particularly important during manufacturing due to the requirement to adhere to GMP guidelines for proper procedures and documentation.
The following example, illustrates how the Dissolution Template may be used to perform a HPLC based dissolution study.
The Dissolution Template captures the workflow outlined in Figure 1 in a single organized electronic document. In order to perform a dissolution study when using SDMS Vision Publisher, a new document is created and the dissolution template is selected. The template consists of six sections (Figure 2): (a) sample and batch information, (b) dissolution bath parameters and sample collection times, (c) reference standard information, (d) dissolution Q-Factor criteria, (e) CDS sample set, (f) dissolution result section, (g) representative chromatograms (optional), and (h) a reference documents summary (i.e., a catalog section listing SOP documents, standard solution preparations, etc.).
Adding links to representative chromatograms is optional (section g above), but may be useful if a dissolution study fails or to highlight degradation products.
To fill out the template, the analyst enters data both manually and by making use of external system interfaces accessed through Vision Publisher to automatically populate specific fields.
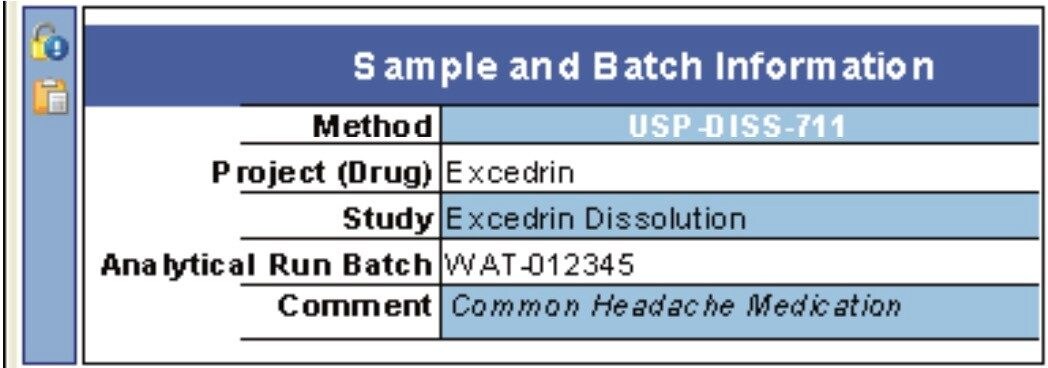
The sample and batch information section will typically consist of the product name, active component, operator, lot/batch number, dissolution apparatus, and dissolution media (see Figures 2 and 3). This information can be entered manually or by using a Laboratory Information Management System (LIMS).
A USP reference standard of the active ingredient is required to determine the amount of drug compound released as a function of time. Typically, the quantity of released active ingredient during an HPLC dissolution study is determined by taking the ratio of the LCUV peak responses for the analyte (Ru) over the standard (Rs) multiplied by some factor, e.g., 2250C (Ru/Rs) where C is concentration.3 Hence, the preparation and the concentration of the standard must be recorded (Figure 4) in order to subsequently perform the necessary calculations.
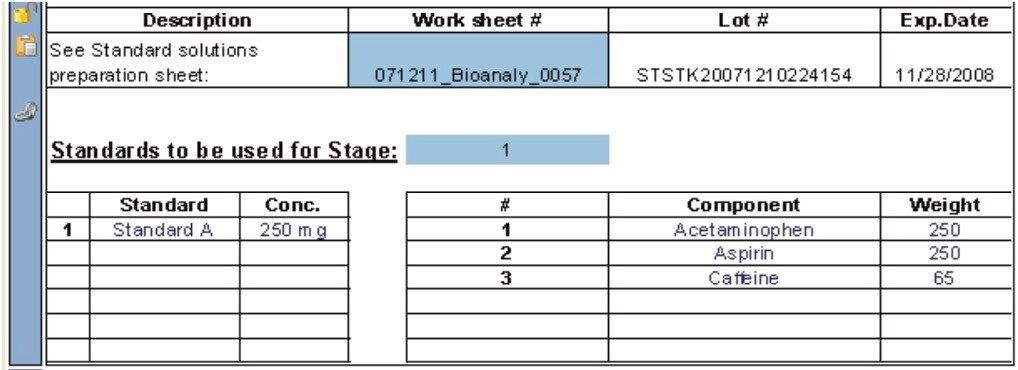
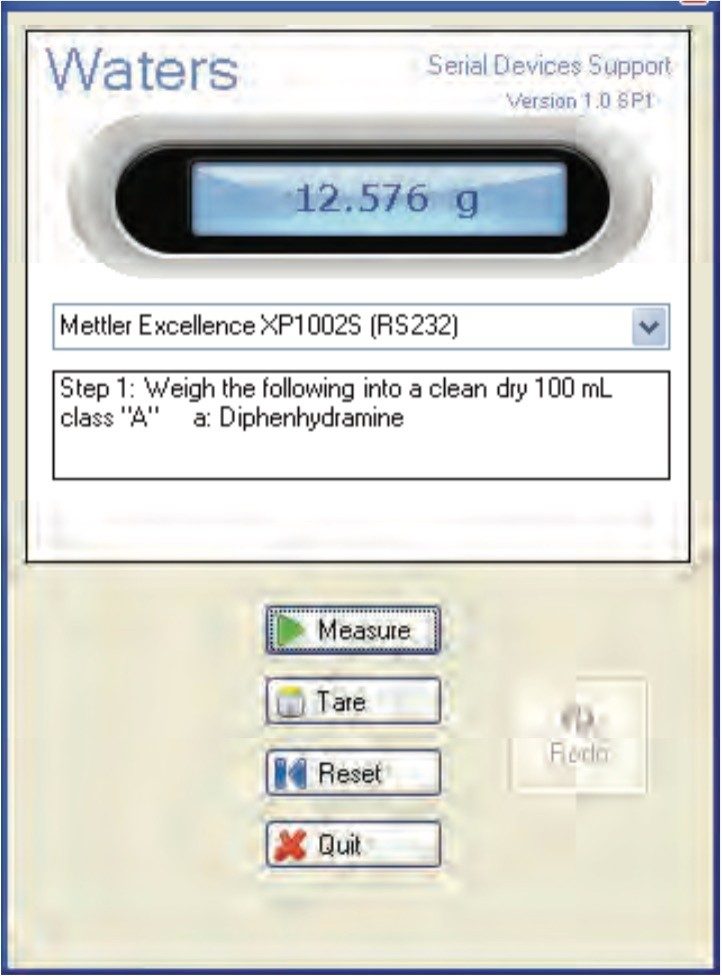
The mass measurements for preparing the USP reference standard (and also for weighing the samples) can be captured automatically within the dissolution document by using an automated balance interface (Figure 5). Hence, accuracy of the measurement is improved since the automated tool eliminates the risk of a transcription error.
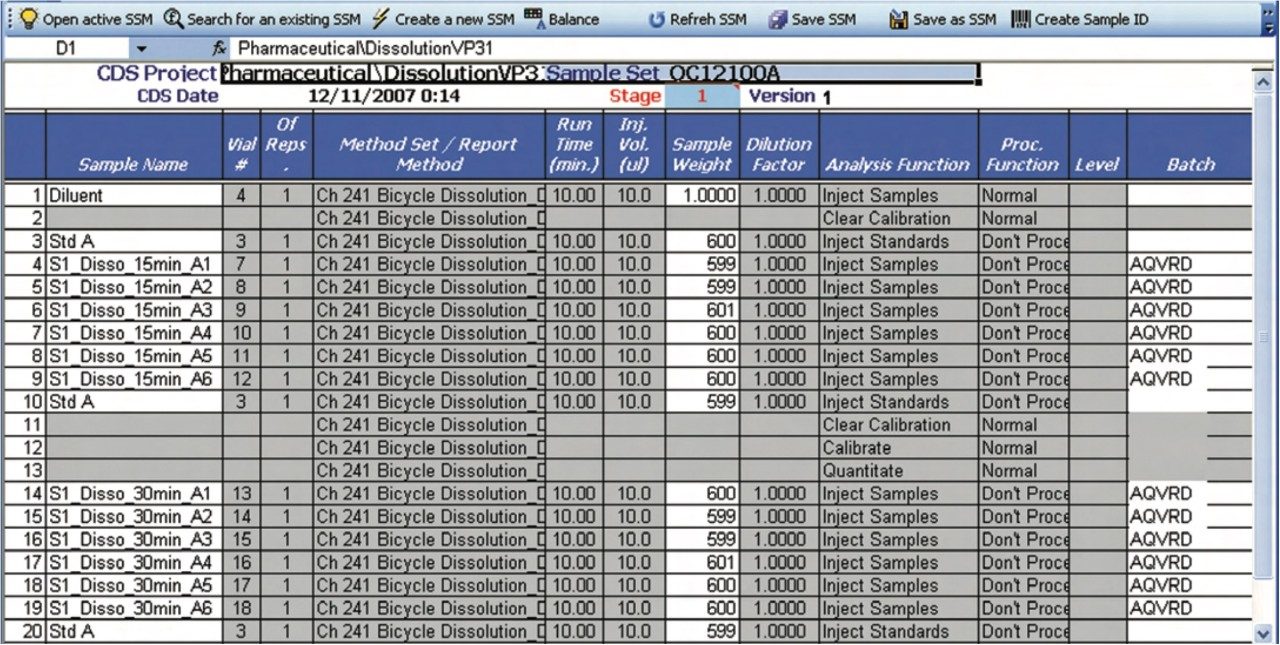
A predefined Empower dissolution sample set method is selected from within the dissolution document. Please note that Empower shown as an example, but the principle is the same for other chromatography systems. Subsequently, the sample and reference standard information are propagated down the sample set (Figure 6). For example, the batch number that was entered into the Sample and Batch Information section of the dissolution document, i.e., the first section of the dissolution document, is now automatically transferred to the sample set. After filling in the required information, the sample set can be submitted for acquisition by the Empower system.
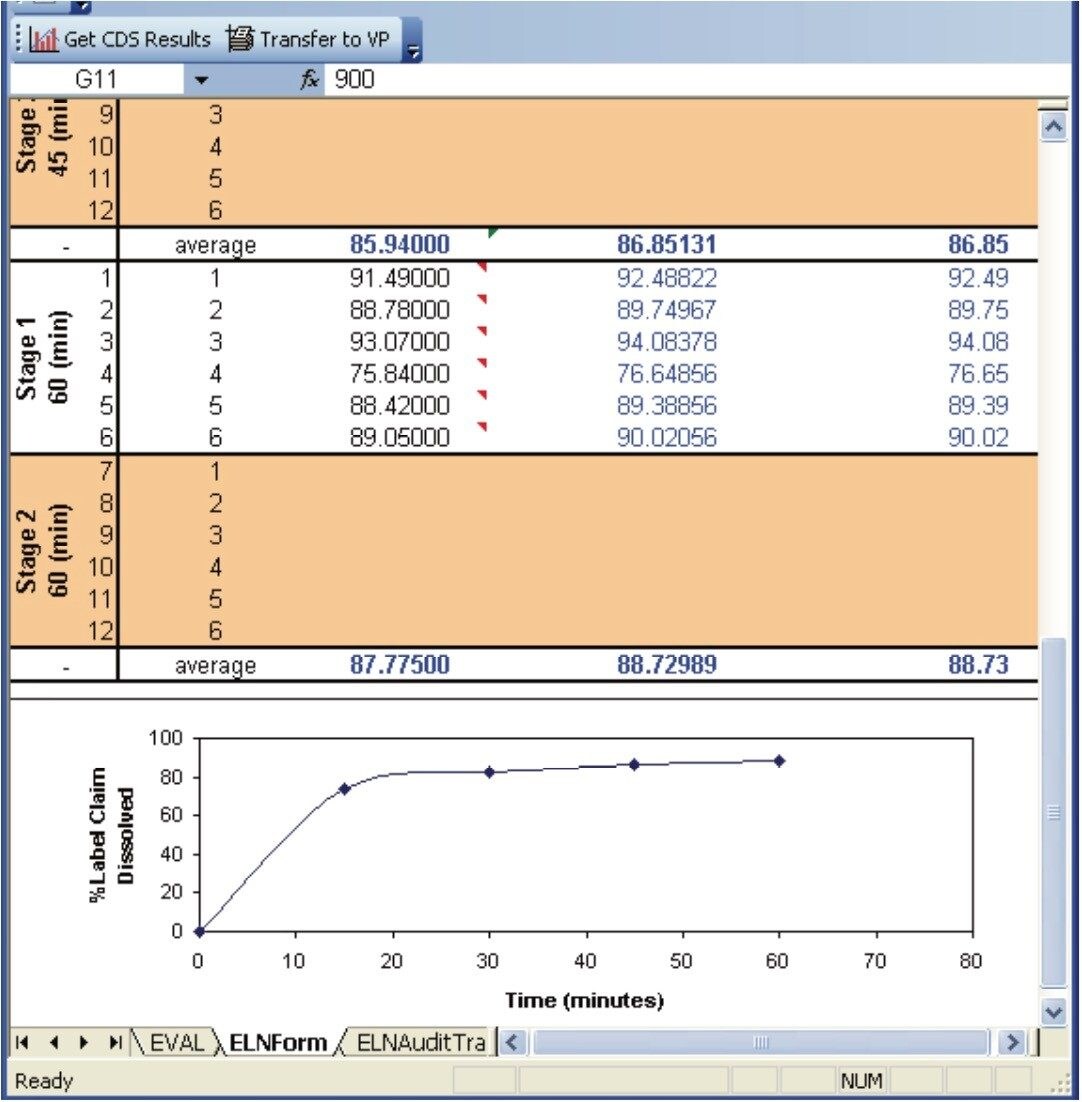
After the data acquisition on the Empower system has been performed, calculations are performed within Empower and the results are then returned to the Dissolution Result Section. These results are simply summarized within the Dissolution Result Section (Figure 8). If necessary, additional calculations and graphing can be performed within the dissolution document by using Microsoft Excel.
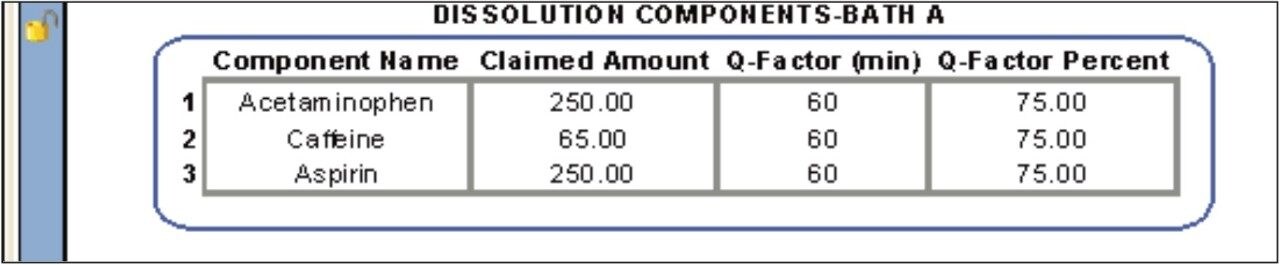
The conformity to compendia acceptance criteria may not be calculated in some chromatography data systems; however, these calculations can be performed within the dissolution document providing an automated means of defining whether an additional dissolution stage is required. For example, the Q-Factor states that at 60 minutes during the dissolution study of Acetaminophen, Aspirin, and Caffeine tablets, 75% of the drug should be released within during Stage 1. If the amount of drug released at 60 minutes doesn’t meet the Q-factor criteria (within an acceptable error) then a Stage 2 dissolution study is required. Figure 7 shows the Q-Factor criteria for the acetaminophen, aspirin, and caffeine tablet dissolution study.5 This functionality allows the analyst to automatically update the CDS sample set to perform additional testing. Three stages are available including Stage 1 (6 replicates), Stage 2 (an additional 6 replicates, and Stage 3 (an additional 12 replicates).
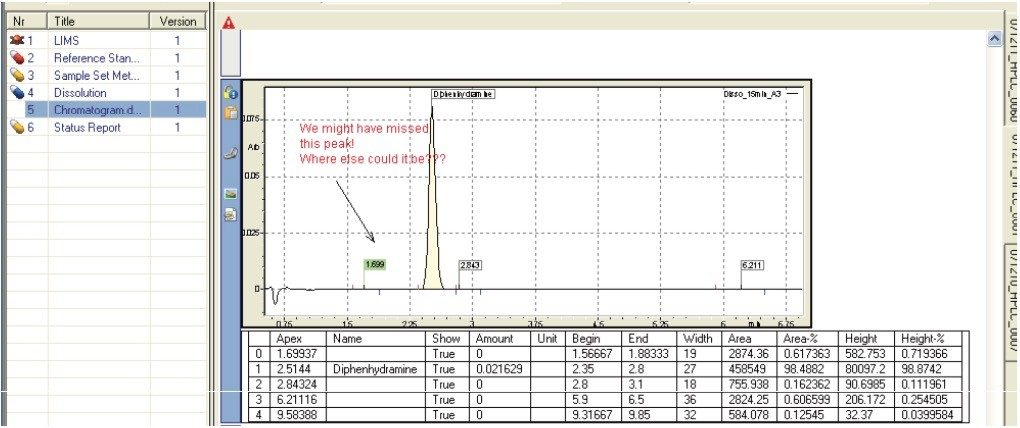
Representative chromatograms may be imported into a dissolution document (Figure 9) in addition to the typical results (e.g., % API released) recorded during a dissolution study. This may be particularly useful to highlight degradation products.
The reference documents summary provides an index of documents that are related to the present dissolution study. This index may include references to the original method, USP reference standard preparation protocols, etc.
The Dissolution Template within SDMS Vision Publisher provides a centralized electronic document to organize important data and results for a dissolution study. Advantages include: (1) direct electronic interface to data sources such as Empower 2, electronic balances, and LIMS; (2) records are searchable and traceable since they are stored electronically; and (3) the electronic record is 21 CFR Part 11 Compliant Ready. Hence, the QC analyst reduces both the time allocated to performing typical dissolution studies, as well as, the risk associated with transcription errors by using the Vision Publisher template.
Dissolution is a critical test for evaluating the performance of drug products. The process comprises a multi-step workflow where accurate documentation of results is an absolute necessity. The Waters Informatics suite provides an integrated solution that can dramatically streamline workflow, increase laboratory productivity, and facilitate product development and release.
720002490, April 2008