This application note demonstrates the advantages of speed and ease of use that self-service UPLC with photodiode array (PDA)/evaporative light scattering (ELS)-MS detection brings to reaction monitoring studies.
Once a chemical hit is found through a library screening process and is verified, optimization of the compounds’ desired properties takes place. This step involves an iterative process of synthesis and reactivity measurement of the new compounds to further develop drug candidates into the lead phase.
Because these reactions may take a long time, chemists need to know as soon as possible if their syntheses are proceeding as desired. This means utilizing measurement capabilities that require minimal sample preparation and provide a fast response giving low detection limits. Another advantageous property might be the ability to measure multiple parameters simultaneously.1
High throughput approaches can provide important time savings in the optimization of process parameters. Open access LC-MS is replacing TLC as a reaction monitoring tool.2 Sample preparation of reaction mixtures can be as minimal as filtering and dilution before injecting into the LC-MS system. This allows fast turnaround of results to allow the chemist to advance to the next step.
The purpose of this application note is to demonstrate the advantages of speed and ease of use that self-service UPLC with photodiode array (PDA)/evaporative light scattering (ELS)-MS detection brings to reaction monitoring studies.

Chromatographic separations were carried out using an ACQUITY UPLC System coupled to an ACQUITY SQ Mass Detector. PDA and ELS signals were collected simultaneously. Samples were analyzed using gradients less than 1 minute. For chromatographic flexibility, a column selection module was added.
|
LC system: |
Waters ACQUITY UPLC System |
|
Column: |
ACQUITY UPLC BEH C8 Column 2.1 x 30 mm, 1.7 μm |
|
Column temp.: |
45 °C |
|
Flow rate: |
800 μL/min |
|
Mobile phase A: |
0.1% Formic acid in water |
|
Mobile phase B: |
0.1% Formic acid in acetonitrile |
|
Gradient: |
5 to 95% B/0.7 min |
|
MS system: |
Waters SQ Detector |
|
Ionization mode: |
ESI positive/ESI negative |
|
Capillary voltage: |
3.0 KV |
|
Cone voltage: |
20 V |
|
Source temp.: |
150 °C |
|
Desolvation temp.: |
450 °C |
|
Desolvation gas: |
L/Hr |
|
Cone gas: |
50 L/Hr |
|
Acquisition range: |
100 to 1300 m/z |
|
Scan speed: |
10,000 amu/sec |
Note: A low volume micro-tee was used to split the flow to the ELS and SQ.
|
Gain: |
500 |
|
N2 gas pressure: |
50 psi |
|
Drift tube temp.: |
50 psi |
|
Sampling rate: |
20 points/sec |
|
Range: |
210 to 400 nm |
|
Sampling rate: |
20 points/sec |
During the compound optimization stage of a discovery cycle, medicinal chemists are not only interested in determining the key structural features responsible for activity and selectivity, but also what structural changes need to be made to improve these characteristics. Because the reactions necessary to bring about these changes may take many steps, chemists need to be sure they are progressing as expected during the course of the reaction synthesis.
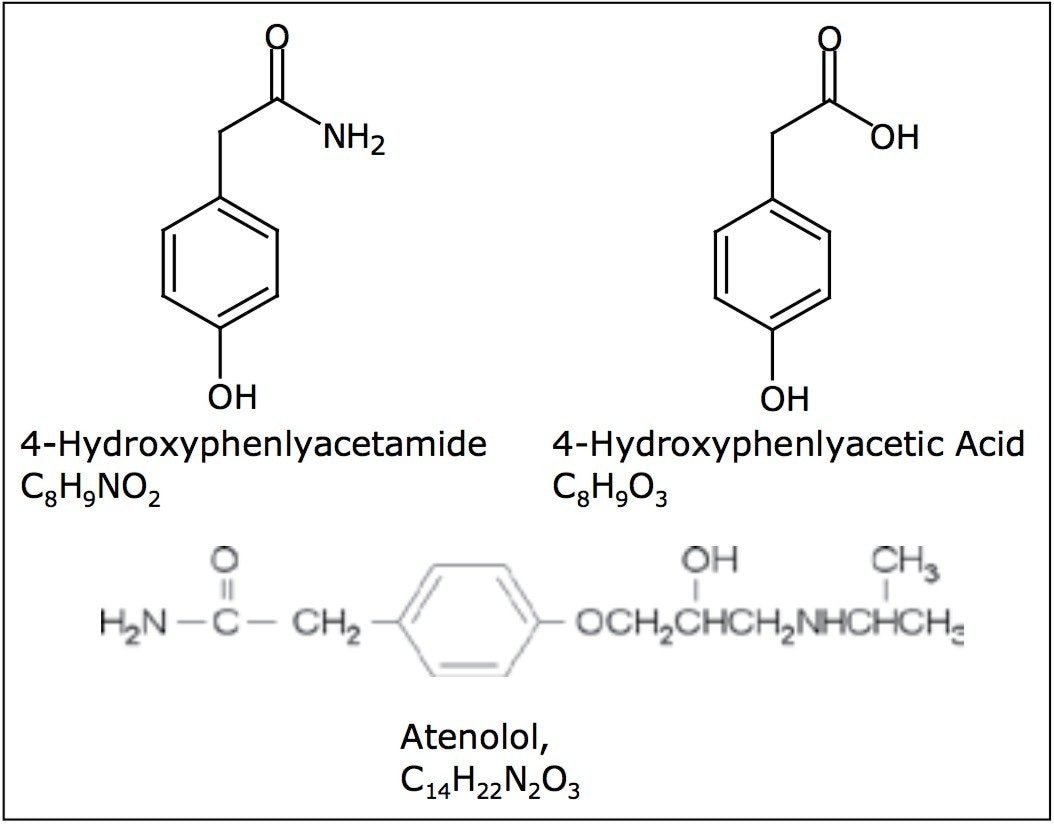
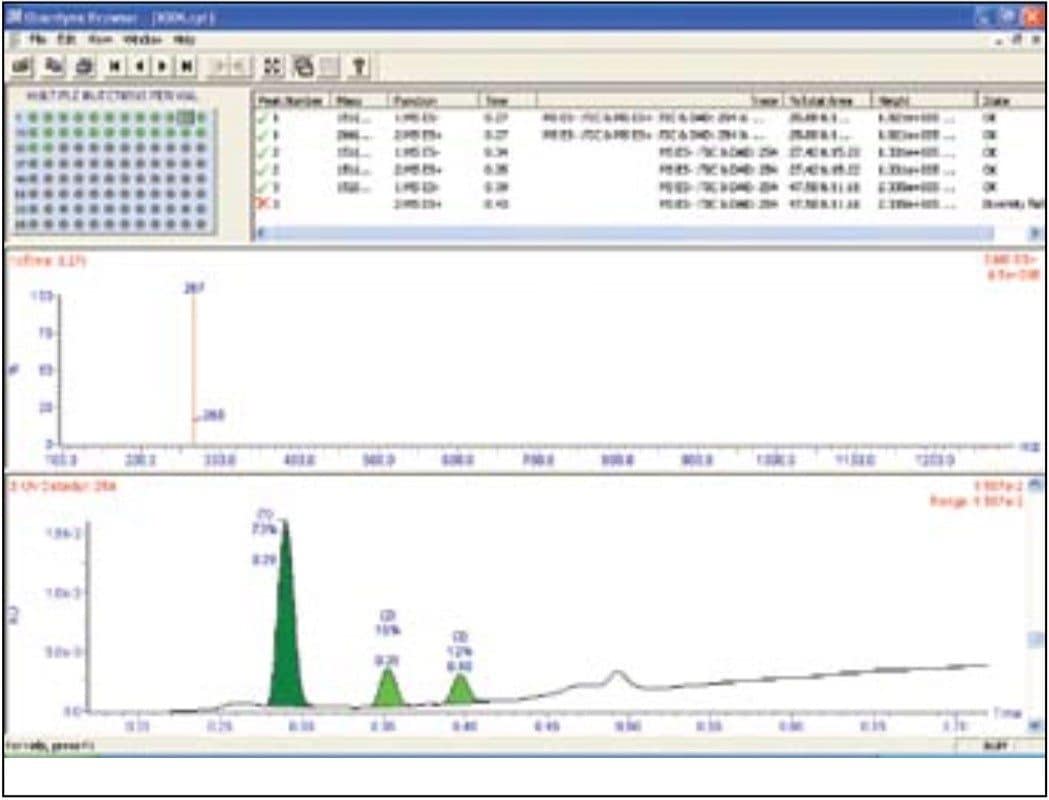
To illustrate the functionality of such a system, the synthesis of atenolol (Figure 2) was used as a reaction model. The increase in the formation of atenolol was monitored, as was the decrease in the intermediate 4-hydroxyacetamide3 (Figure 3). A reaction by-product 4-hydroxyphenylacetic acid was also observed.
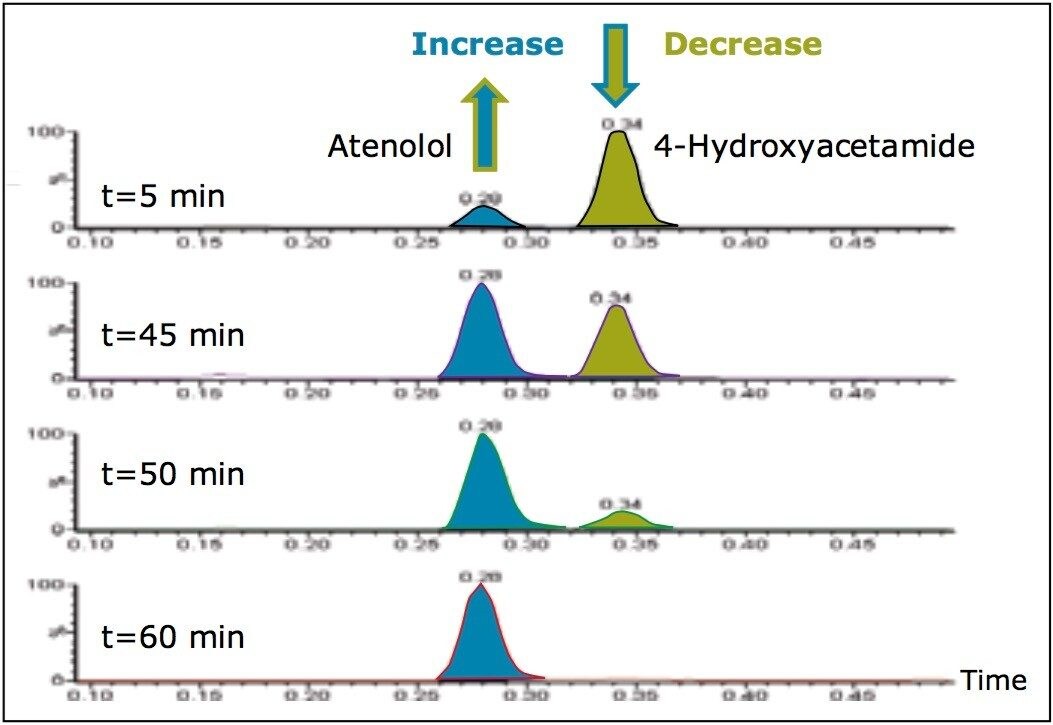
The ACQUITY SQD is capable of scan speeds of up to 10,000 amu/sec. Consequently, it is possible to employ a large number of scan functions in a single run while still maintaining adequate peak characterization. The fast scan speed is essential for this functionality, as peak widths of 1 second or less are common with the use of UPLC. Scanning multiple functions allows confirmation of compound synthesis to be obtained on reaction components whether they ionize in positive ion mode or negative ion mode, ESI or APCI. The total cycle time of the method was 1 minute 20 seconds, facilitating increased sample throughput.
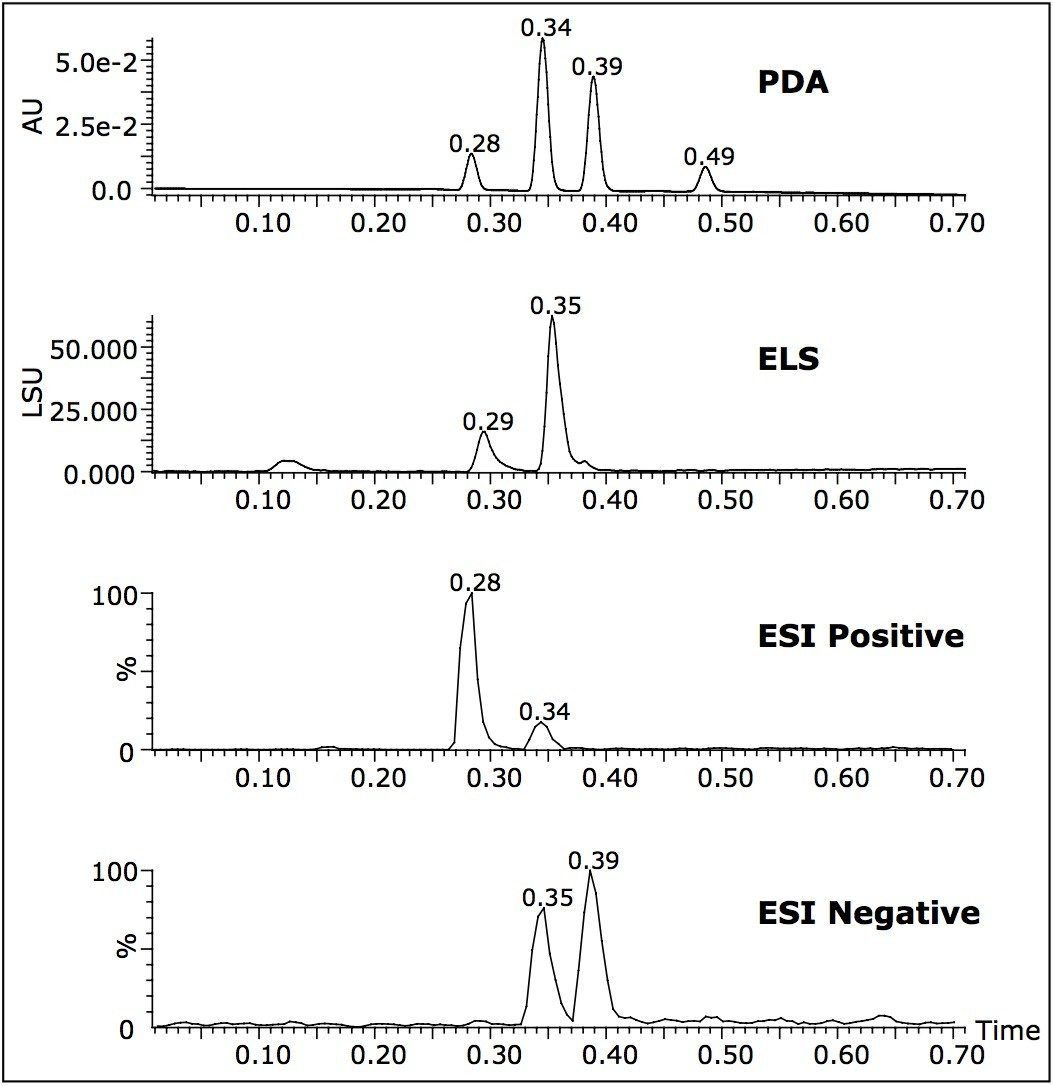
A single run can also provide UV spectral information and an estimation of compound purity at low wavelengths. ELS detection is based upon the degree to which solute particles scatter light. It has been known to give rise to similar responses for related compounds.4 The signal can give a tentative estimation on the relative quantities of the components present (Figure 4). It is also an alternative detector to UV, which depends on the presence of a chromaphore. As can be seen from Figure 4, atenolol ionizes in ESI positive ion mode (retention time 0.28 min). The reaction intermediate 4-hydroxyphenylacetamide ionizes in both positive and negative ion mode (Rt 0.34 min) and 4-hydroxyphenylacetic acid (Rt 0.39 min) only ionizes in negative ion mode.
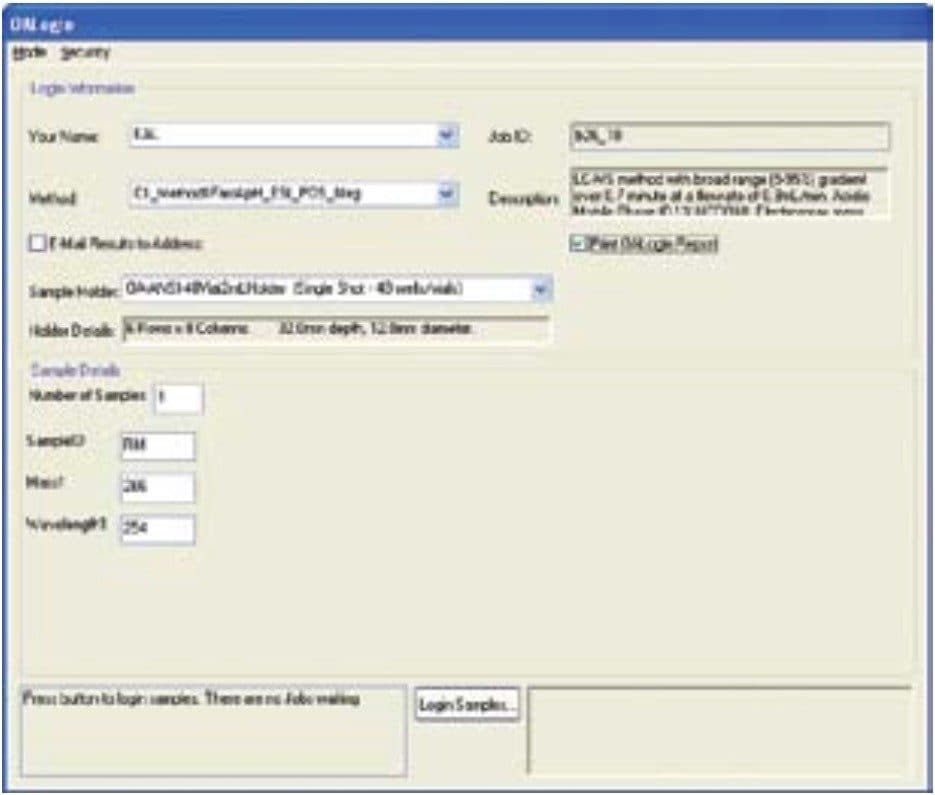
The OpenLynx Open Access Application Manager, part of MassLynx Software, allows chemists to walk up to a terminal and log in samples while entering the minimum information required to run the samples.
The OpenLynx OA Login screen shown in Figure 5 allows the administrator to set up the system such that the user only needs to input the information requested, and then upon completion, select the Login Samples button. This will either tell the user the designated autosampler position, or confirm the position that the user has chosen, and ask for confirmation of position before it will run the sample. In addition to a simplified sample submission process, the OpenLynx Application Manager can then process data automatically and produce a summary report that can be emailed or printed as desired.
The information contained in the summary report is viewed via the OpenLynx browser shown in Figure 6. It clearly defines what components are found or not found. Chromatograms and spectra are generated based on the processing parameters set up by the administrator in the OpenLynx method.
During the compound optimization stage of a discovery cycle, medicinal chemists are not only interested in determining the key structural features responsible for activity and selectivity, but also what structural changes need to be made to improve these characteristics. Because the reactions necessary to bring about these changes may take a long time, chemists need to be sure they are progressing as expected.
By using a walk-up UPLC-MS system, chemists were able to quickly and easily monitor their reactions, noting the relative amounts of starting materials and products. They were also able to note the formation of any side products and make necessary alterations to their reaction protocol to minimize these.
The described system and software combination can autonomously evaluate large numbers of samples, with a cycle time of 1 minute 20 seconds. Data can then be automatically processed and a summary report can be generated. The scan speed capabilities of the ACQUITY SQD make it possible to better characterize narrow chromatographic peaks. This has become a necessity since the advent of sub-2 μm particle technology, where chromatographic peaks can be 1 second wide or less. The fast scan speed allows the chemist to extract as much data as possible per injection by switching between APCI and ESI as well as positive and negative ion modes.
Open Access gives the chemist a walk-up system that is flexible for analytical data acquisition. It runs as a complete system, from sample introduction to end results.
The use of the fast-scanning MS along with the throughput of UPLC technology allows the chemist to obtain high quality and comprehensive data about their compounds in the shortest possible time. This combined with intelligent open access software allows informed decisions to be made faster, thus supporting the needs of the modern drug discovery process.
720002258, June 2007