SIMI: A Different Approach to Revealing Dangerous Subvisible Impurities
Waters Corporation, United States
Published on November 06, 2025
Introduction
Foreign particulate matter, also known as extrinsic particles, has become a serious threat to patient safety in recent years, with cases linked to severe immunogenic reactions, deaths, and costly recalls.1 In 2021 alone, Takeda recalled 1.6 million doses of the Moderna COVID-19 mRNA vaccine in Japan after several patient deaths were linked to steel particulates shed from a malfunctioning pump.2 Over the span of seven years, from 2014–2017, there were more than 110 drug recalls as a result of glass delamination,3–4 and several more due to remnant crystals,5 owing to the difficulty of identifying the presence and source of foreign particulate matter. To prevent against such threats, manufacturers require high throughput technologies to better identify foreign particulate matter in real time.
Results and Discussion
Shining a Light on Extrinsic Particles with SIMI
Side illumination membrane imaging (SIMI) is a proprietary illumination, imaging, and analysis technique exclusively available to Aura instruments. Utilizing the principles of darkfield microscopy,6 SIMI is conducted on an Aura membrane plate using an oblique illumination source which emits light at an angle parallel to the surface of the plate.
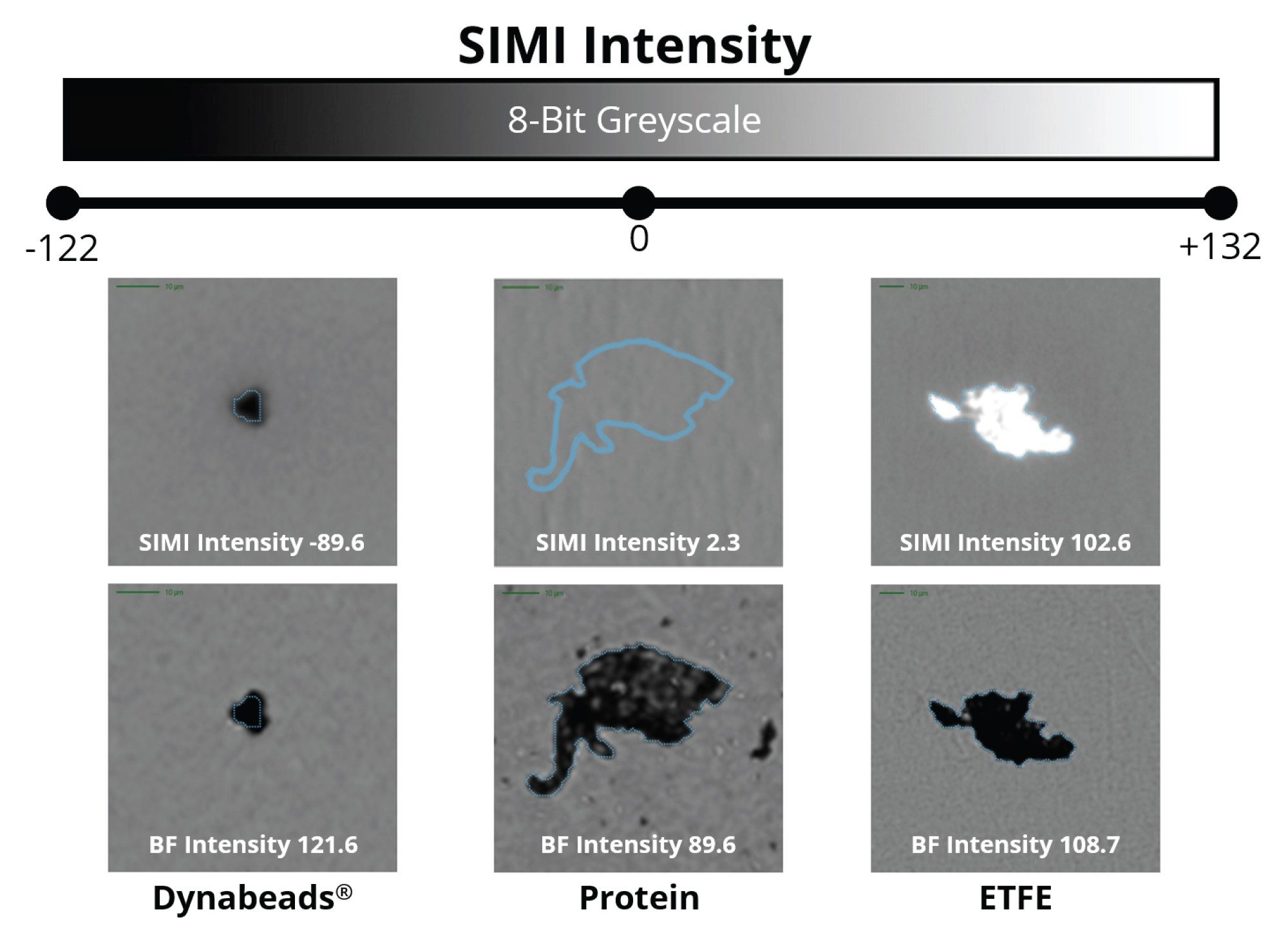
Compared to backgrounded membrane imaging (BMI), which utilizes brightfield microscopy (epi-illumination) where particles scatter the incident light isotropically (i.e., at all angles), side illumination membrane maging (SIMI) is a technique that is specifically designed to detect light scattering from high refractive index particles, as well as those that protrude out of the plane from the membrane surface. A particle’s average SIMI intensity is measured in a relative scale where the inherent SIMI scattering of the membrane is defined as SIMI = 0. Positive SIMI values indicate particles protruding out of the membrane, as is the case with microplastics, fibers, and other common extrinsic particles, whose average SIMI intensities vary from 0–255. On some occasions, like with metallic particles and absorbing oils, particles will have reduced light refraction relative to the membrane and thus display negative SIMI values, varying from 0 to -122.
The majority of foreign, extrinsic particles exhibit a high refractive index and are mechanically rigid in structure, causing them to protrude from the membrane surface and scatter side illumination to a high degree, ideal for SIMI analysis. In contrast, biologic particles exhibit the opposite properties—low refractive index and malleable structures, causing them to lie flat with minimal side scatter profiles, which is best analyzed with BMI or fluorescence membrane microscopy (FMM). Utilizing all three microscopy approaches is most ideal to facilitate the determination of all extrinsic (non-biologic) and inherent (biologic) particles in biologic formulations.
Using SIMI to Distinguish the Indistinguishable: Extrinsic Particles
Protein Aggregates vs. Ethylene Tetrafluoroethylene
The National Institute of Standards and Technology (NIST) created a protein aggregate mimetic reference material made from ethylene tetrafluoroethylene (ETFE). ETFE (NIST RM 8634) particles were designed to have similar morphological features, refractive index, and size distribution of common sub-visible protein aggregates.7 Using standard brightfield illumination, ETFE particles are indistinguishable from protein aggregates in a mixed population.8 The addition of the SIMI channel enables the rapid and accurate differentiation of plastic (rigid) from protein (flat, soft) particles.
Biological aggregates, such as protein and viral vectors, are often mechanically compliant and thus lay flat on the surface of the membrane after filtration (Figure 1). Coupled with low refractive index (proteins have similar optical properties as water), their SIMI signature is very weak and often indiscernible from the membrane, as evidenced in Figures 1b and 2b.
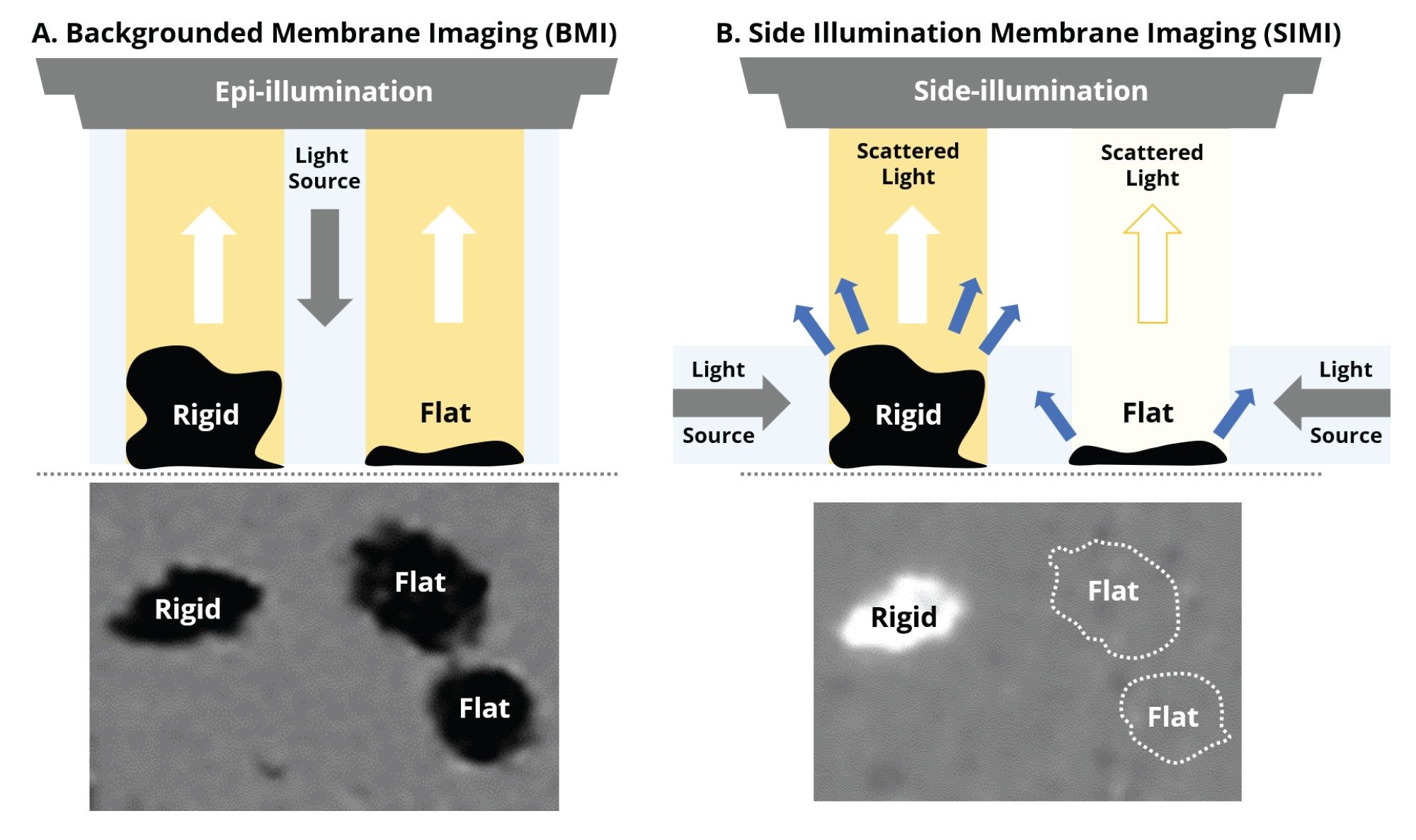
In Figure 2, we show subvisible IgG protein aggregates imaged with both BMI and SIMI. BMI provides high contrast images of protein aggregates appearing as dark particles, while in the SIMI channel they are virtually invisible.
While ETFE particles display similar high refractive index contrast to protein aggregates, they appear morphologically and optically similar. However, ETFE’s rigid nature makes these particles exhibit bright scattering signatures in the SIMI channel (Figure 3). As extrinsic and inorganic particles sit high on the membrane after filtration, distinguishing extrinsic particles is both facile and rapid, avoiding the requirement for complex machine learning algorithms that are often required in flow-based techniques.
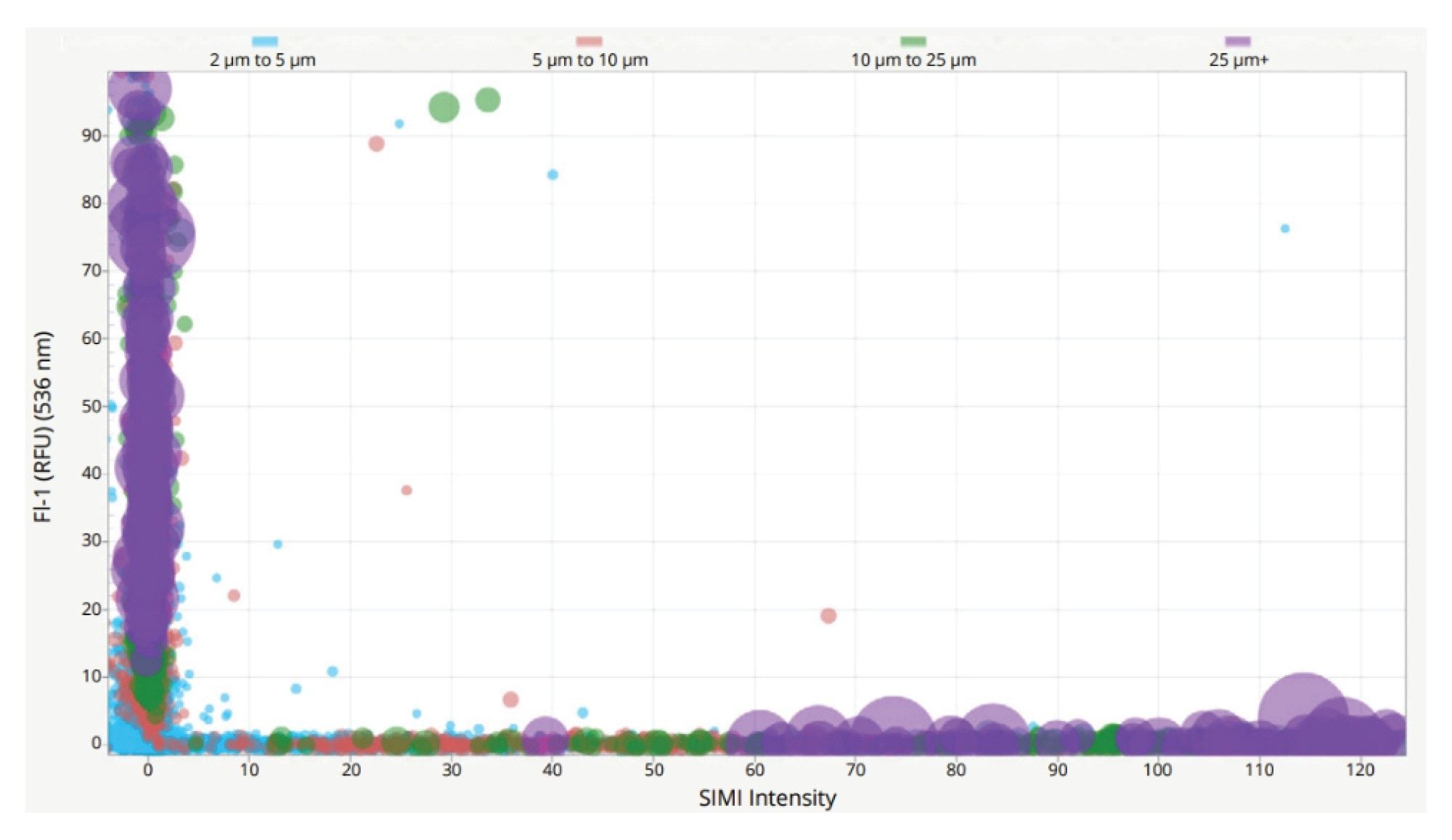
To highlight the increased side-scattering of rigid materials, a solution containing a mix of ETFE and IgG aggregates was used, which was fluorescently stained with thioflavin T (ThT), and detected using FMM. When used in conjunction with SIMI, it is possible to define subpopulations on Aura instruments using appropriate characteristics for both extrinsic particles (ThT -ve; SIMI +ve) and protein aggregates (ThT +ve; SIMI -ve) as visualized in Figure 4.
Particles Shedding from Common Biological Containers
Glass Particles
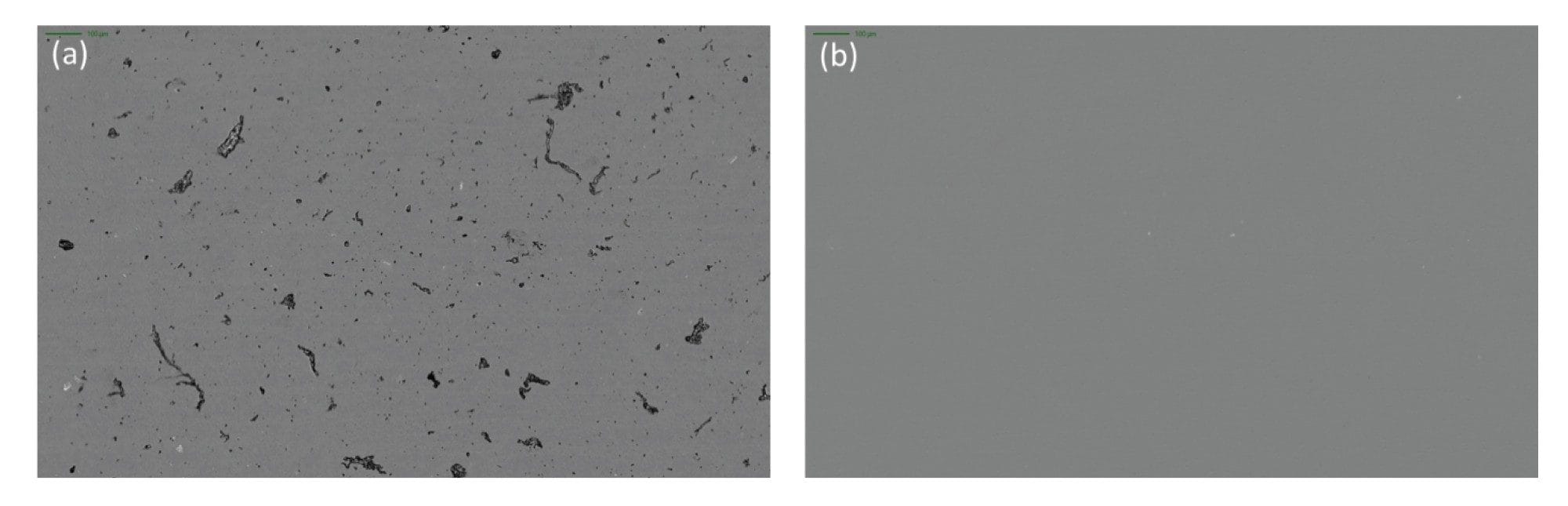
Glass particles, often generated from defective vials, are considered dangerous subvisible particles due to their ability to puncture capillaries and accumulate in tissues. In fact, this is one of the leading causes of product recalls.3 They present a significant analytical challenge as glass particles are optically transparent and have low refractive index similar to commonly used drug medias (n = ~ 1.5). Flow based technologies like flow imaging microscopy and light obscuration (LO) have inherently low refractive index contrast (water refractive index, n = 1.33 making detecting these particles nearly impossible. Aura System utilizes BMI and SIMI to effortlessly detect glass particles, thanks to the inherent high contrast imaging (Figure 5a) and strong side scattering due to the inherent rigidity of glass (Figure 5b) provided by separating the particles of interest from the media it is in.
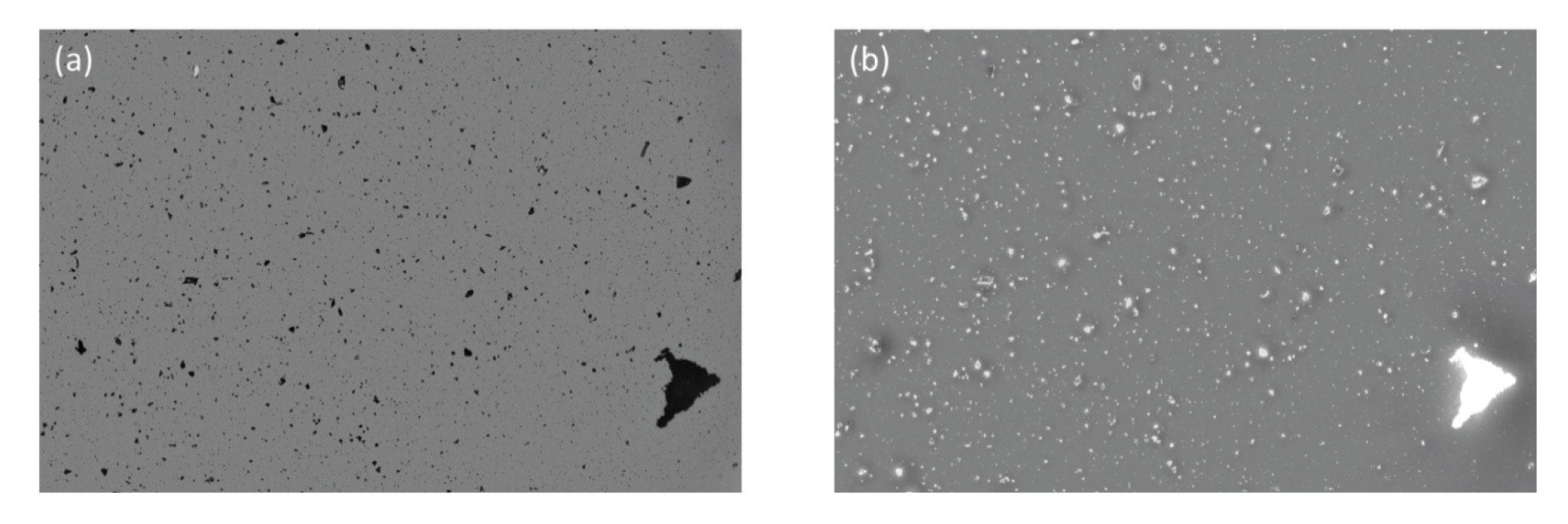
Polypropylene Particles
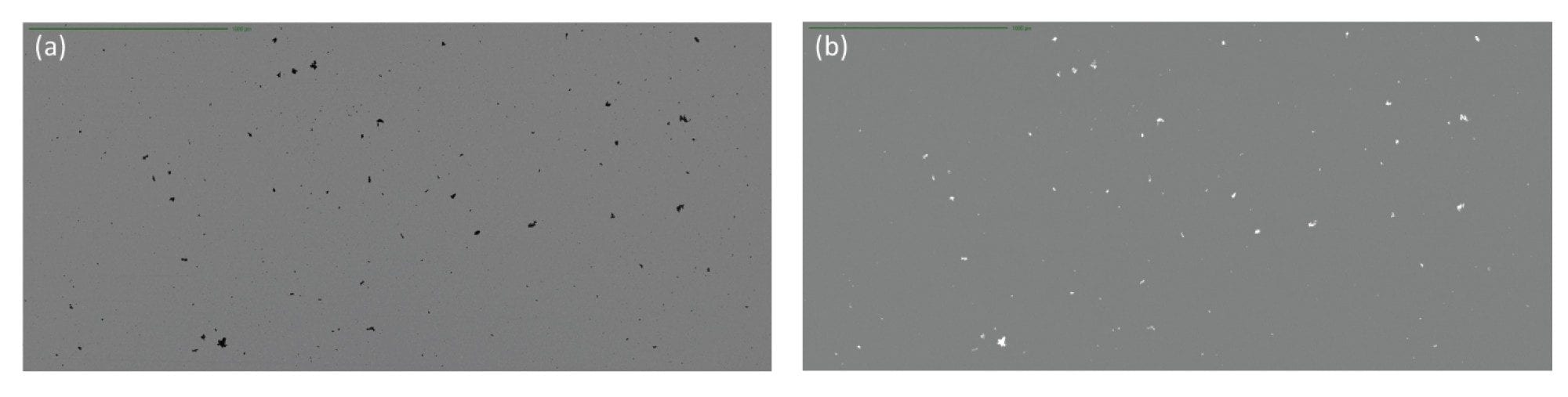
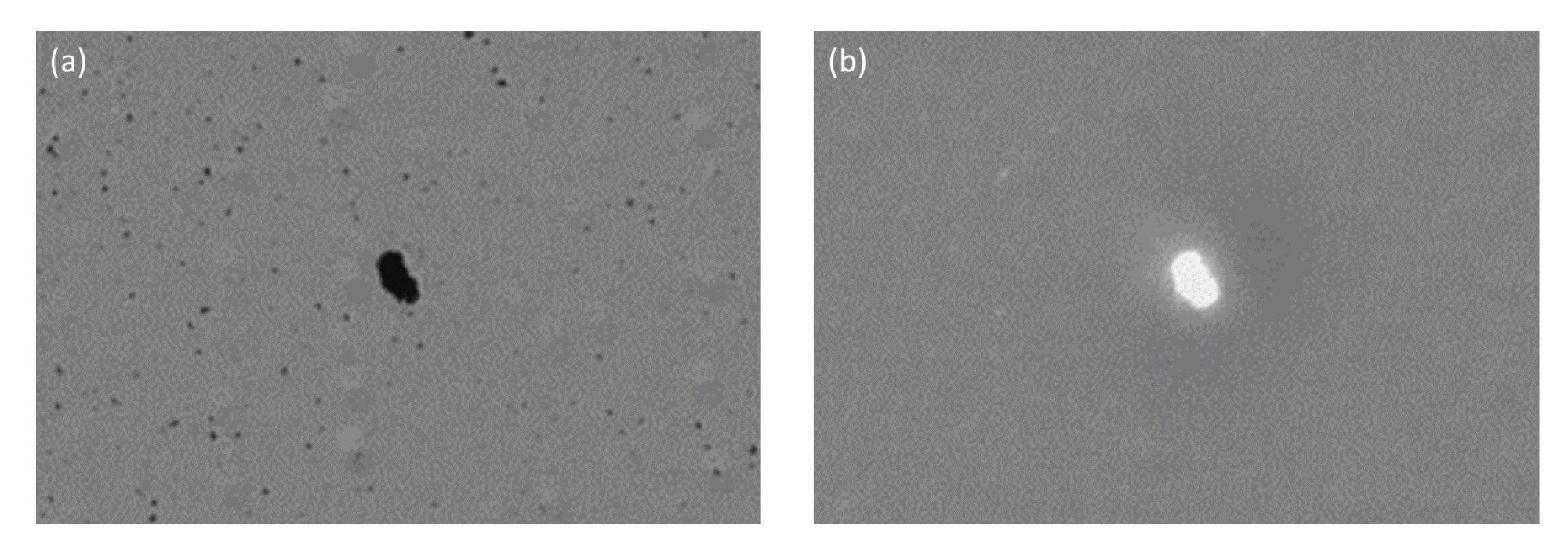
Another common source of contaminating extrinsic particles is those generated from a drug vial, container or tubing. For example, threaded polypropylene vials tend to shed many subvisible particles, which are distinguishable by their SIMI signature. Particles greater than 5 µm equivalent circular diameter (ECD) represent significant side scatter and thus are easily detected (Figure 6). This is essential to properly identify the source of subvisible particles.
Other Extrinsic Contaminants
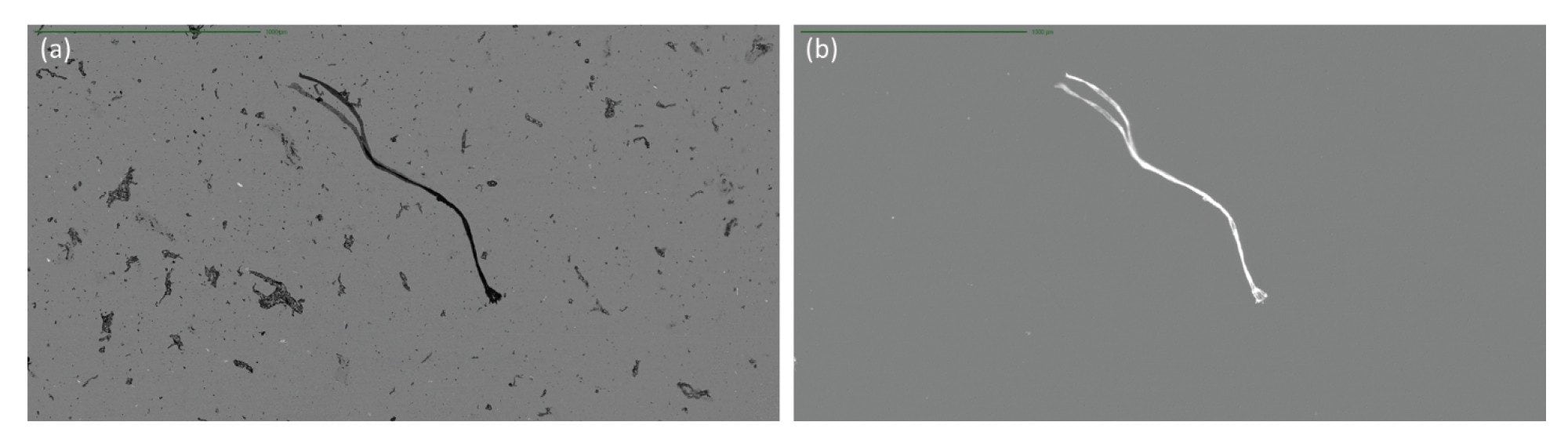
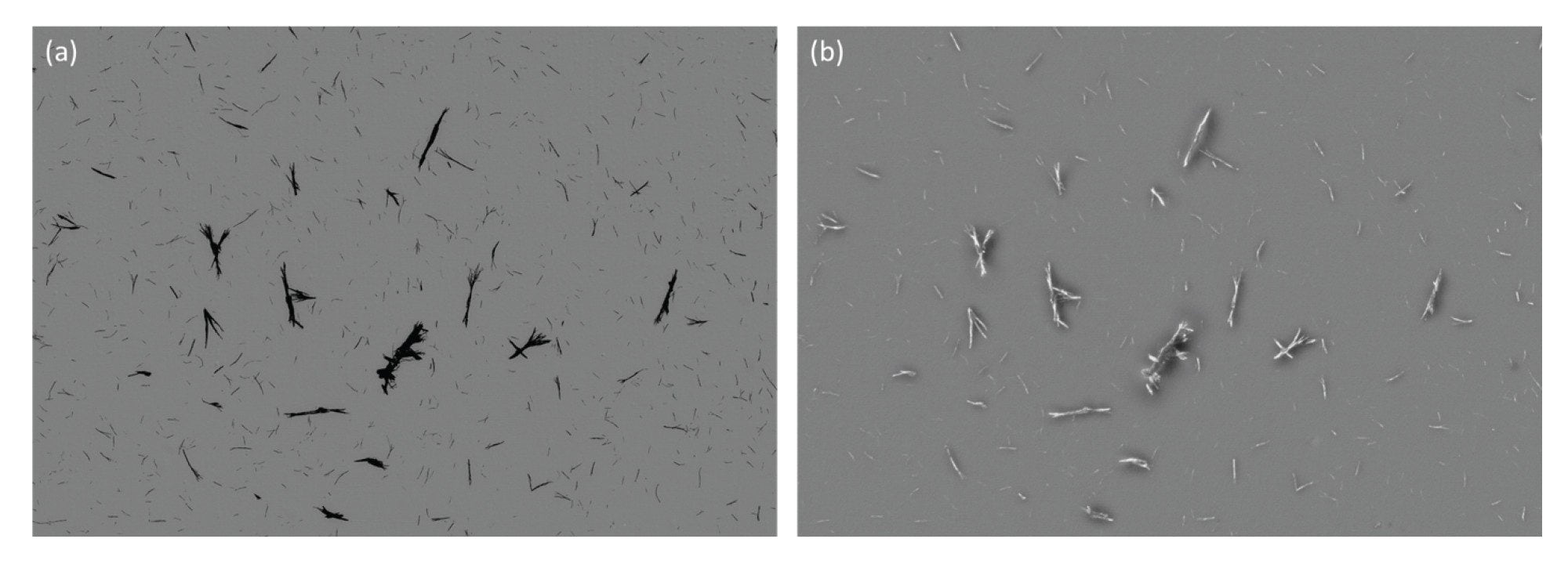
Extraneous Fibers
The complexity of biologics manufacturing results in the increased potential for extraneous materials and fibers contaminating the final drug product. Fibrous subvisible particles, including those generated from clothing, filtration membranes, and syringe filters, all within the manufacturing environment, have two key identifying features, rod-like morphology defined by a low aspect ratio and strong SIMI intensity. Extraneous contaminants compare very favorably against inherent particles derived from the drug product when using SIMI as the distinguishing feature (Figure 7).
Residual Dynabeads
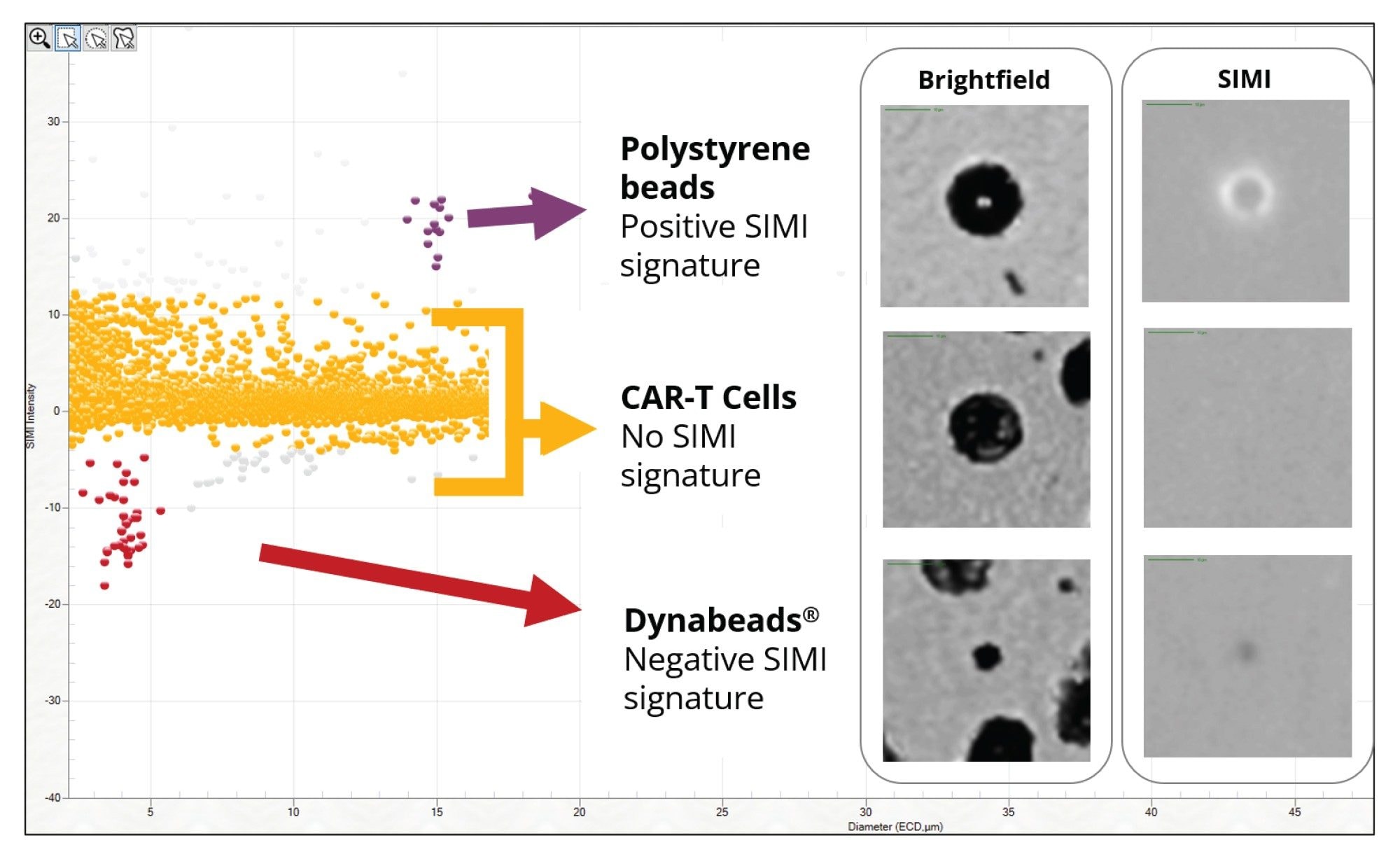
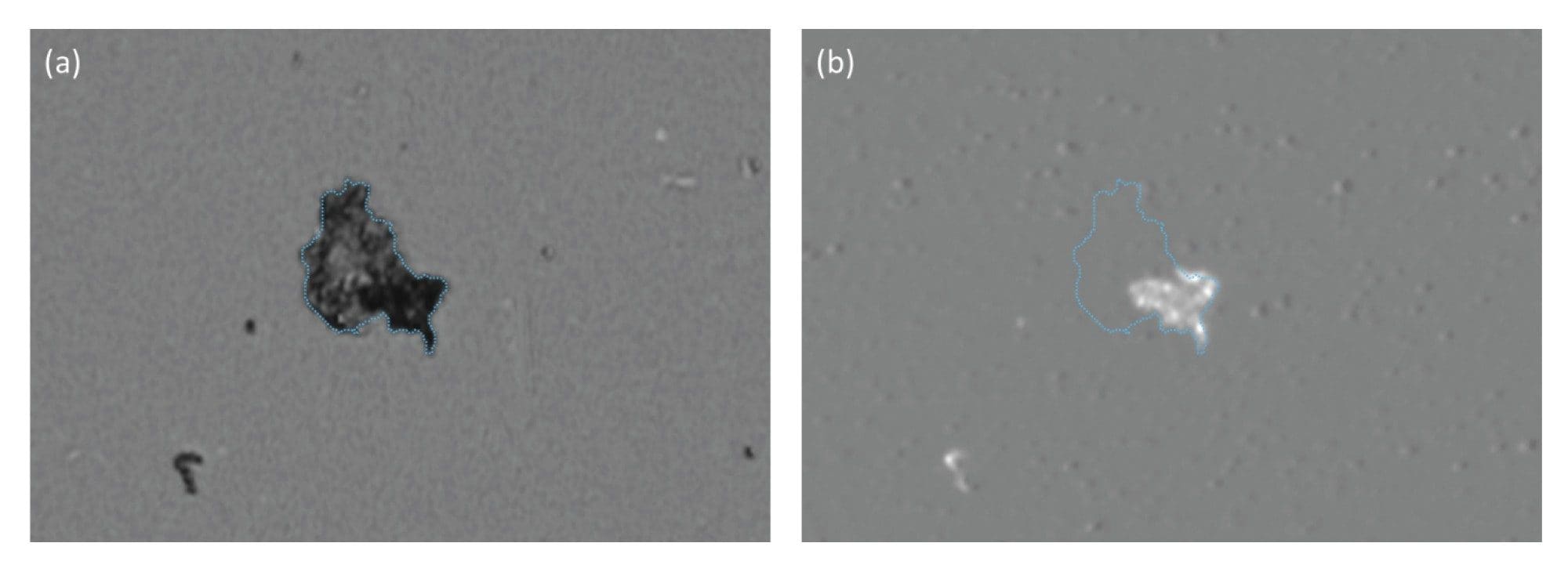
In certain instances, as a key part of the biomanufacturing process, extrinsic particles may be intentionally added to drug product and removed prior to product release. One such instance is the addition of CD3+/CD28+ Dynabeads™ (Thermo Fisher Scientific®) to stimulate the activation of CAR-T cells to drive clonal expansion. These magnetic beads are subsequently removed from the product, however residual Dynabeads populations have the potential to present an immunogenic risk to the patient.
Current methods for quantifying residual Dynabeads rely on painstaking manual hemocytometry. Additionally, distinguishing Dynabeads from cells or cell debris is a significant challenge due to the high cell concentrations present in cell therapies, creating a longstanding challenge in CAR-T cell quality control. Aura systems can use morphology, sizing, and SIMI analysis to identify these ”needle in a haystack” particles. The relative SIMI signature of Dynabeads, on a white plate, result in a negative SIMI profile which enables their distinction from the CAR-T cell therapy and other extraneous particles, including polystyrene beads (Figure 8). In addition, Aura instruments have the ability to perform particle analysis (counting and sizing particles) directly in SIMI mode to count Dynabeads directly without having to worry about the presence of high cell concentrations, as expanded in Tech Note 4.
Using SIMI to Distinguish the Indistinguishable: Intrinsic/Inherent Particles
Small Molecule Crystals
The ability to define subpopulations of particles derived from the inherent drug product is an important distinction. We have previously demonstrated how this can be achieved in biologic drug formulations, however in small molecule therapies being able to determine crystal forms of the API is critical. It is possible to characterize the insoluble particles as either amorphous or crystalline by combining both BMI and SIMI. Small molecule crystals have very characteristic angular rod like morphology and exhibit strong SIMI signature, compared to amorphous particles (Figure 9).
Embedded Extrinsic and Intrinsic Particles
The complex relationship between drug product, excipients, and other particles can result in significant mixing of distinct types of particulate matter in a biopharmaceutical sample. Particles, be they extrinsic or intrinsic/inherent to the therapeutic, can provide nucleation sites for aggregation. The combination of BMI and SIMI can help elucidate occurrences of biological material interacting with non-biological particles (Figure 10). Composite particles, like these, are challenging to characterize using flow imaging systems or systems without multiple light sources and detection capabilities. The SIMI module in Aura instruments provides an additional dimension to analyzing extrinsic and intrinsic particle mixes.
Conclusion
SIMI is a unique approach to providing additional characterization of subvisible particles and is available in all Aura models to help rapidly identify foreign material in biological samples with a high degree of specificity. A key tertiary imaging mode in addition to BMI and FMM, SIMI provides selective, orthogonal morphological information to identify tall and rigid materials, a defining feature of most extrinsic particles (Figure 11). While particle sizing and counting is done via BMI, SIMI provides illumination, imaging, and analysis that can be useful across a variety of biological products to reveal foreign subvisible particles from the earliest to the latest of stages in the bioprocess.
References
- Jiao, N., et al. Characterization of Subvisible Particles in Biotherapeutic Prefilled Syringes: The Role of Polysorbate and Protein on the Formation of Silicone Oil and Protein Subvisible Particles After Drop Shock. Journal of Pharmaceutical Sciences. 109(1): 640–645. https://doi.org/10.1016/j.xphs.2019.10.0662.
- Japan: 1.6 Million Moderna Vaccine Doses Recalled; Novavax Replacement Deal Signed. Pharmaceutical Technology. https://www.pharmaceutical-technology.com/comment/moderna-vaccine-recalled-novovax-replacement/
- https://www.scribd.com/document/630784333/SiO2-Glass-Related-Recalls-in-Pharma-pdf
- Mylan and Hospira Both Recall Injected Drugs After Particulate Discovered In Vials. Fierce Pharma. https://www. fiercepharma.com/manufacturing/mylan-and-hospira-recall-different-drugs-after-particulate-discovered-vials
- Hospira Recalls Cancer Drug After Finding Carboplatin Crystals. FDA News. https://www.fdanews.com/articles/152219-hospira-recalls-cancer-drug-after-finding-carboplatin-crystals?v=preview
- What Is Darkfield Microscopy?. Evident. https://www.olympus-lifescience.com/en/discovery/what-is-darkfieldmicroscopy/
- Development and Interlaboratory Evaluation of a NIST Reference Material RM 8366 for EGFR and MET Gene Copy Number Measurements. The National Institute Standard and Technology. https://www.nist.gov/publications/development-and-interlaboratory-evaluation-nist-reference-material-rm-8366-egfr-and-met
- Kiyoshi, M., et al. Collaborative Study for Analysis of Subvisible Particles Using Flow Imaging and Light Obscuration: Experiences in Japanese Biopharmaceutical Consortium. Journal of Pharmaceutical Sciences 108(2): 832–841. https://doi.org/10.1016/j.xphs.2018.08.006
720009094, October 2025