Maximizing Protein A Column Performance, Lifetime, and Reliability Through Clean-in-Place
Emery Domain, Philip Wuthrich, Steve Shiner, Stephan M. Koza
Waters Corporation, United States
Published on December 05, 2025
Abstract
The useful lifetime of protein A analytical columns in bioprocessing applications can vary quite drastically depending on method conditions and sample composition. A common failure mode for such columns is fouling caused by the build-up of cell culture media components. This application note demonstrates how employing best practices can help mitigate these potential problems to realize the longest possible column lifetimes. Results were obtained through constant repeat injections of a simulated chinese hamster ovary (CHO) bioreactor sample while monitoring each column for signs of degradation in terms of backpressure increase and chromatographic performance. Clean-in-place (CIP) injections were performed intermittently to achieve well over 1,500 injections for both 2.1 x 20 mm and 3.9 x 5 mm column configurations.
Benefits
- Analytical Protein A Column lifetimes > 1,500 injections
- Injection-based CIP protocol
- Robust protein A resin with high chemical and mechanical stability
Introduction
Analytical protein A affinity columns are employed in upstream bioprocessing to monitor monoclonal antibody (mAb) titer output, often during the early stages of development, where media components can be abundant compared to the product of interest. Protein A columns selectively capture mAbs from cell culture media and consequently are exposed to a myriad of media components, including host-cell proteins, nucleic acids, and other cellular debris. While the majority of these matrix materials pass through the resin unbound, a small portion remains affixed to the mAb or otherwise bound non-specifically to the column. Over time, these remnants can accumulate and begin to affect column performance. Notable signs of affinity column degradation, indicating the end of a column’s lifetime, include backpressure increase, peak shape deterioration, and loss of recovery and/or column capacity.1,2
Column fouling can be minimized with proper sample preparation techniques, particularly clarification, filtration, and optional in-line filtration ( p/n: 205002586), however smaller media components are inevitably injected onto the Protein A Column. To this point, a proper CIP protocol is necessary to control the build-up of these undesired species.3 It is also worth noting that fouling by media-related contamination is not the only cause of column failure in Protein A Affinity chromatography. Microbial contamination is also a particular concern for protein-based chromatography due to the volume of aqueous mobile phases. Unclean mobile phase can lead to system and column contamination, which manifests in similar column degradation symptoms. Fortunately, problematic bacterial growth can be avoided through hygienic mobile phase preparation and handling, frequent system decontamination and proper column storage.4,5 Waters suggested best practices to mitigate microbial contamination during analytical chromatography with aqueous buffers can be found in application notes 720004182 and 720007077.
Additionally, as with any chromatographic media, Protein A Column lifetime is impacted by the chemical stability of the resin and the mechanical stability of the packed bed. This is an especially important consideration for protein A materials which are routinely exposed to both low and high pH solutions during sample elution and CIP protocols.2 The column lifetime experiments described herein aim to probe the cumulative effects of media fouling, pH cycling, and continuous high-pressure injections.
In these experiments, BioResolve™ Protein A Affinity Columns of both configurations were challenged with repeated injections of a non-transfected, clarified cell culture media sample spiked with rabbit IgG to mimic real-time analysis of bioreactor samples. Intermittent injections of NISTmAb in 2x phosphate buffered saline (PBS) were performed to analyze chromatographic performance, and a CIP protocol was then implemented to extend column lifetime. Peak shape, recovery, and column pressure were monitored throughout the course of experimentation, and dynamic binding capacity was compared pre- and post-lifetime analysis.
Experimental
Sample Description
- NISTmAb reference material, RM 8671 (10 mg/mL)
- CHO conditioned media (clarified, 0.2 µm filtered, ~90% viability, with ~0.04 mg/mL trastuzumab)
- Rabbit IgG Affinity Purified, Innovative Research (26.3 mg/mL)
LC Conditions
|
LC system: |
ACQUITY™ H-Class Bio UPLC System with Quaternary Solvent Manager (QSM), Flow-Through Needle Sample Manager (FTN), and CH-A Column heater |
|
Detection: |
ACQUITY UPLC TUV Detector, 5 mm titanium flow cell Wavelength: 280 nm Sample Rate: 80 points/sec |
|
Columns: |
BioResolve Protein A Affinity Column, MaxPeak Premier, 3.5 µm, 2.1 x 20 mm (p/n: 186011369) BioResolve Protein A Affinity Column, MaxPeak Premier, 3.5 µm, 3.9 x 5 mm (p/n: 186011379) |
|
Column temperature: |
Ambient |
|
Sample temperature: |
6 °C |
|
Injection volume: |
Cell culture media: 10 µL NISTmAb: varied |
|
Loading mobile phase: |
Dulbecco’s Phosphate Buffered Saline (DPBS) at 2x concentration, pH 7.4 (0.2 µm sterile-filtered) |
|
Elution mobile phase: |
100 mM KH2PO4 and 100 mM KCl, pH 3.0 |
|
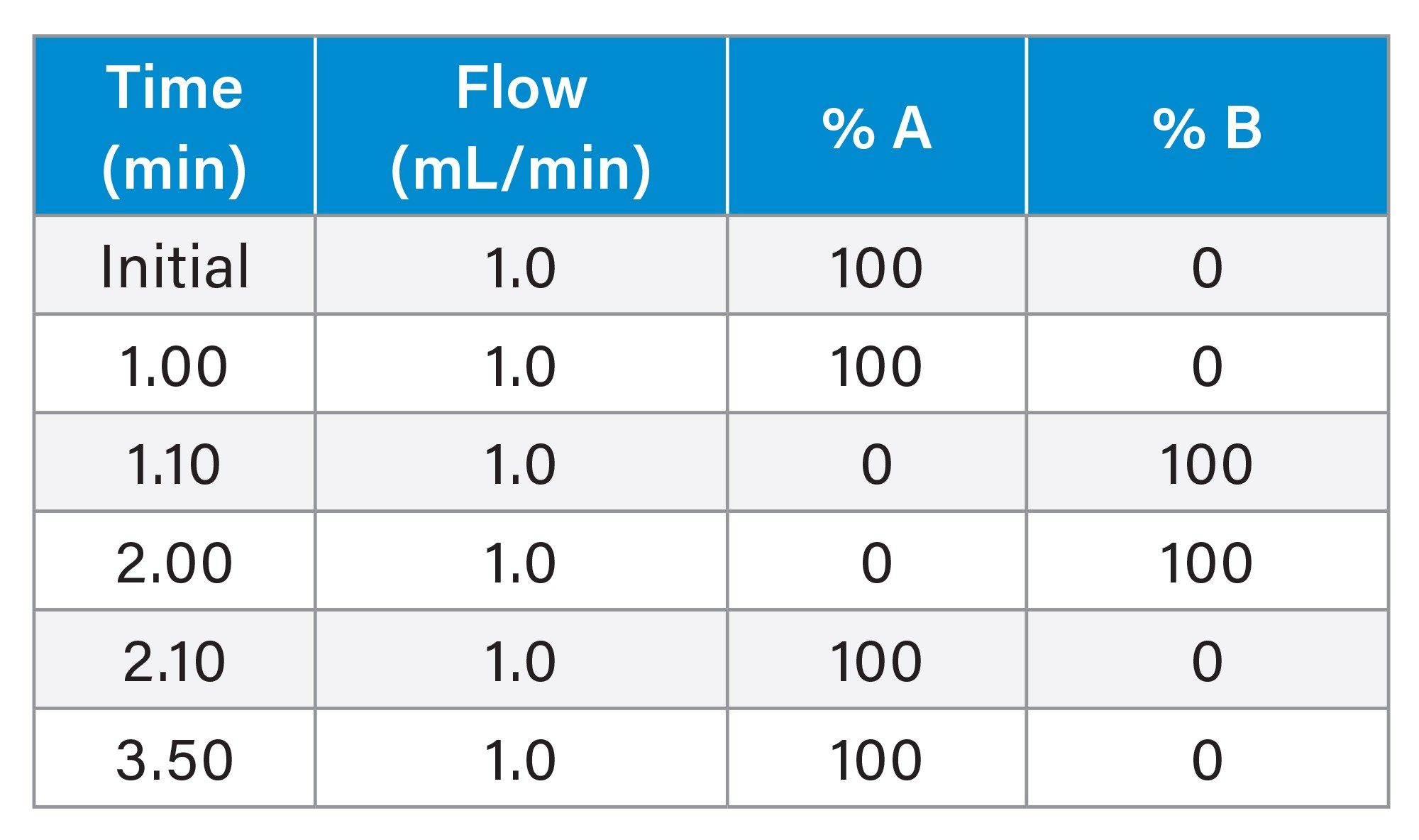
Flow rate: |
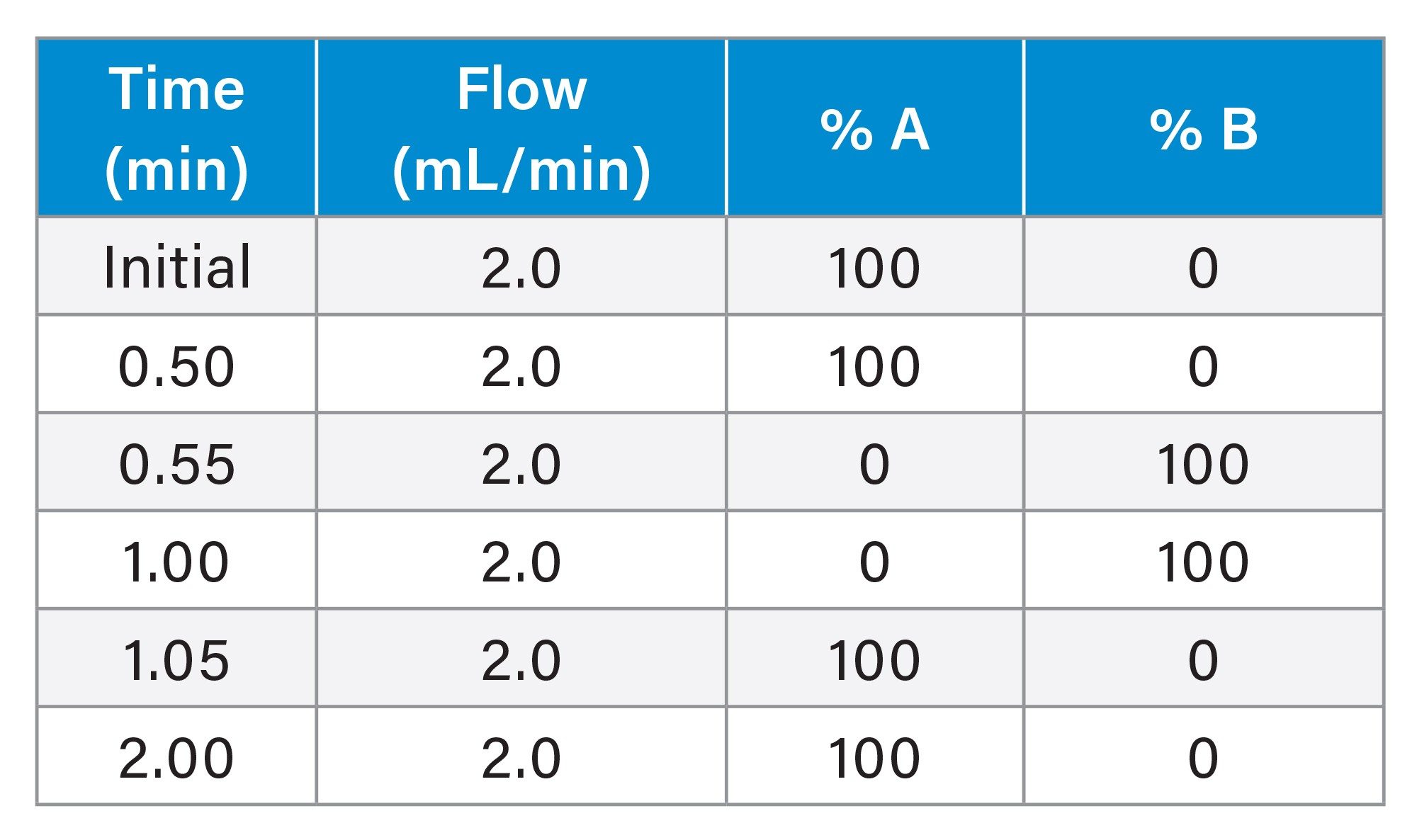
2.1 x 20 mm: 1.0 mL/min 3.9 x 5 mm: 2.0 mL/min |
|
Data management: |
Empower™ Chromatography Data System |
2.1 x 20 mm Gradient Table
3.9 x 5 mm Gradient Table
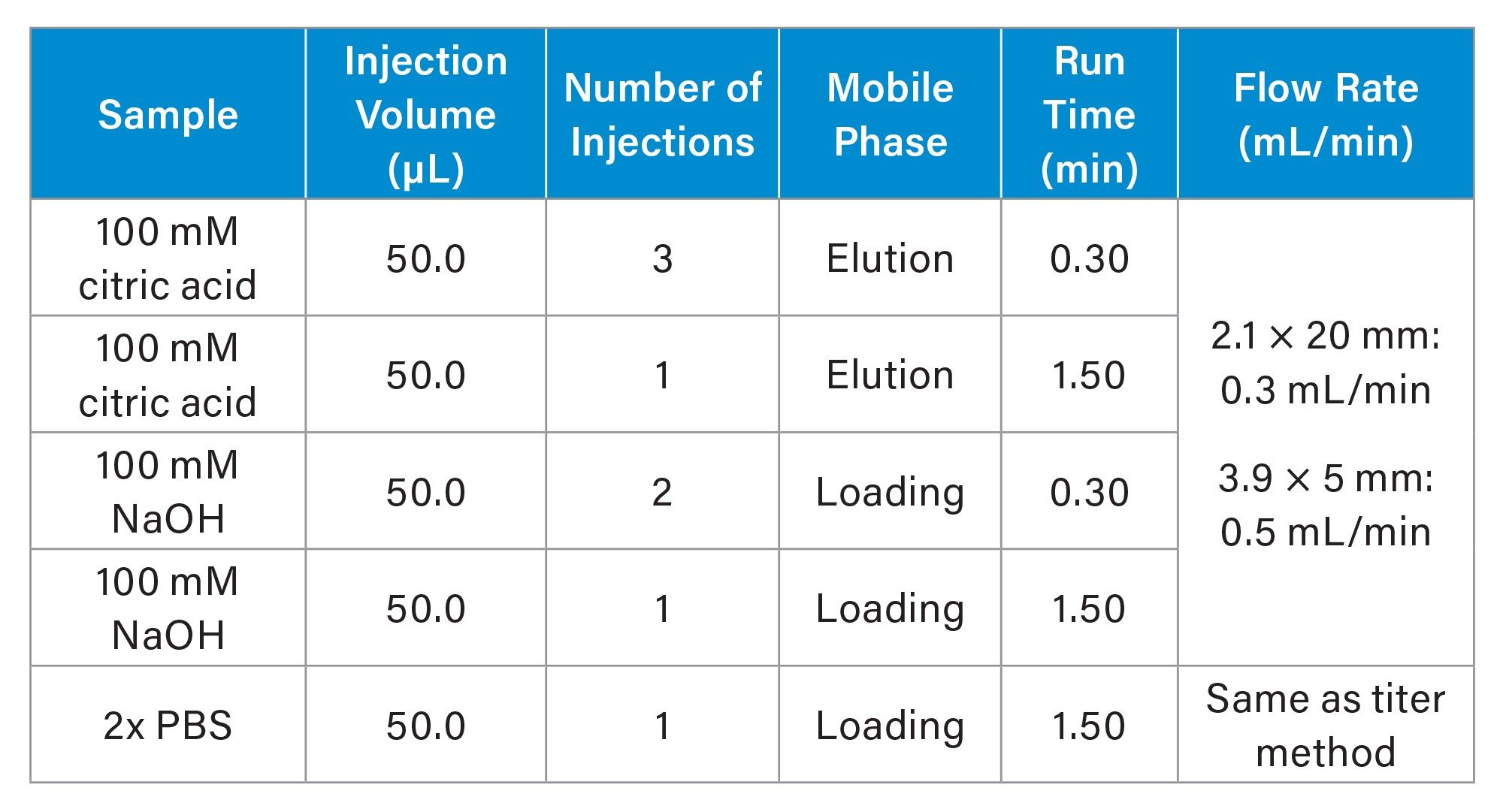
CIP Protocol
Results and Discussion
Baseline column performance for the 2.1 x 20 mm configuration in the absence of any cleaning procedure was first established. Non-transfected CHO cell culture media was 0.2 µm filtered then spiked to 1 mg/mL with rabbit IgG. The inclusion of the polyclonal rabbit IgG ensured that the proteins on the resin surface underwent repetitive conformational changes associated with binding and elution. Following every 200 injections of spiked media, a three-point calibration curve was built using injections of NISTmAb diluted in PBS, and the 1 µg injection was used to assess peak shape and recovery. Column backpressure was monitored during every injection, both spiked media and NISTmAb in buffer. After 500 injections of spiked media sample without a cleaning protocol, the column demonstrated an 18.3% increase in backpressure with no decline in peak shape, recovery, or dynamic binding capacity.
To lengthen column lifetime and maintain appropriate backpressure, an injection-based CIP method was developed. This CIP protocol involves four 50 µL injections of 0.1M citric acid in elution buffer, followed by three 50 µL injections of 0.1M sodium hydroxide in loading buffer. Flow rate for the CIP injections was reduced from 1.0 mL/min to 0.3 mL/min, to both maintain a reasonable column backpressure and to increase the residence time of the cleaning solutions on the column. The final step of the CIP protocol is a single 50 µL injection of PBS at the typical running flow rate of 1.0 mL/min in this instance, for the 2.1 x 20 mm column. In contrast to the benchmarked column lifetime of ~500 injections without cleaning, the implementation of this CIP protocol every 50 media injections enabled the column to achieve 1,000 injections with only a 10.2% increase in backpressure and no deleterious effects on chromatographic performance. Additional media injections were then appended to this CIP-based lifetime experiment to test the column until failure, defined in this case as a 20% backpressure increase or with similar metrics surrounding recovery and column capacity. Continuing to implement the CIP protocol every 50 injections, the 2.1 x 20 mm column exhibited a 19.9% increase in pressure following 1,500 spiked media injections. Though the column backpressure increase approached the internal limit of 20%, the performance of the column remained stable with robust peak shape, recovery, and dynamic binding capacity at the conclusion of this lifetime challenge.
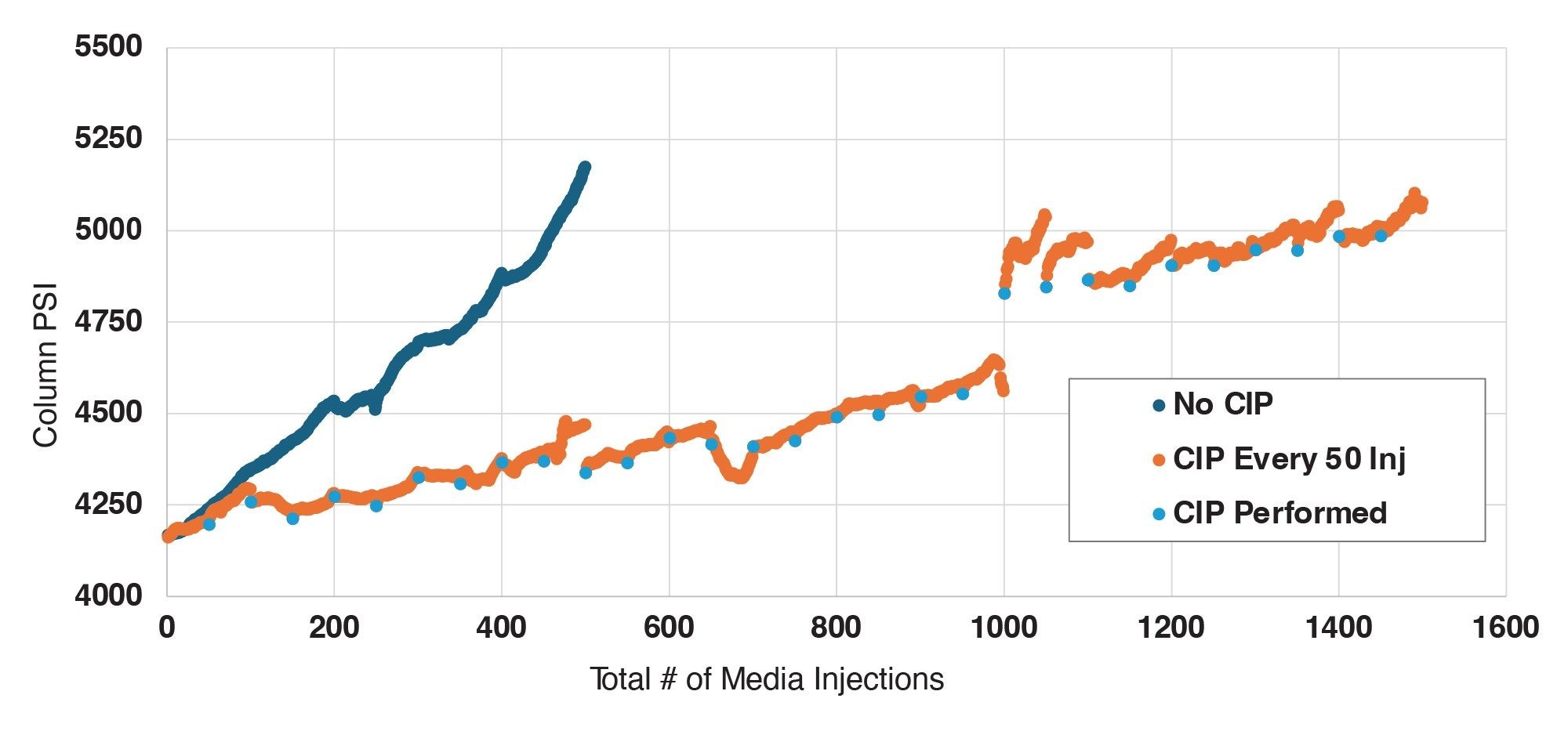
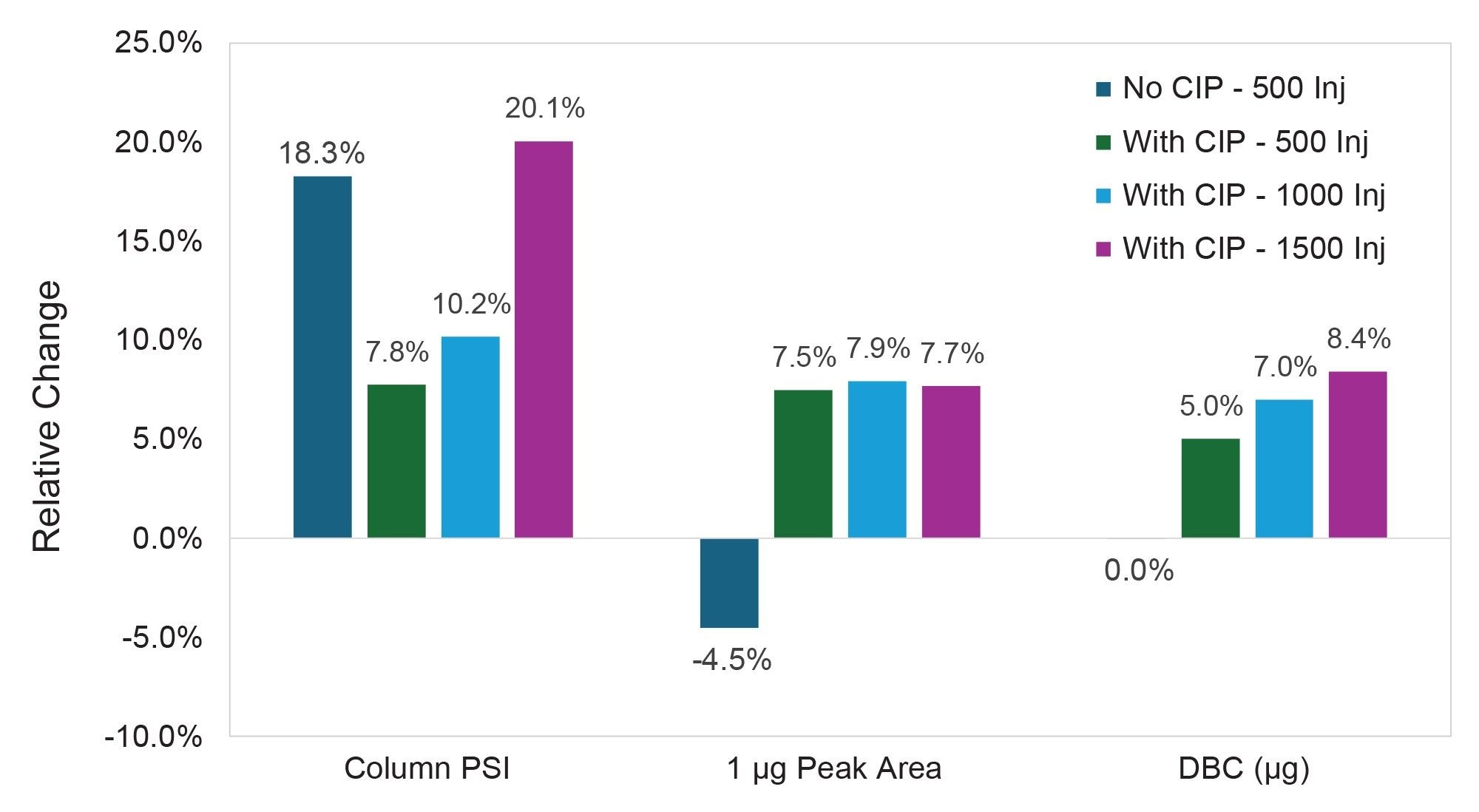
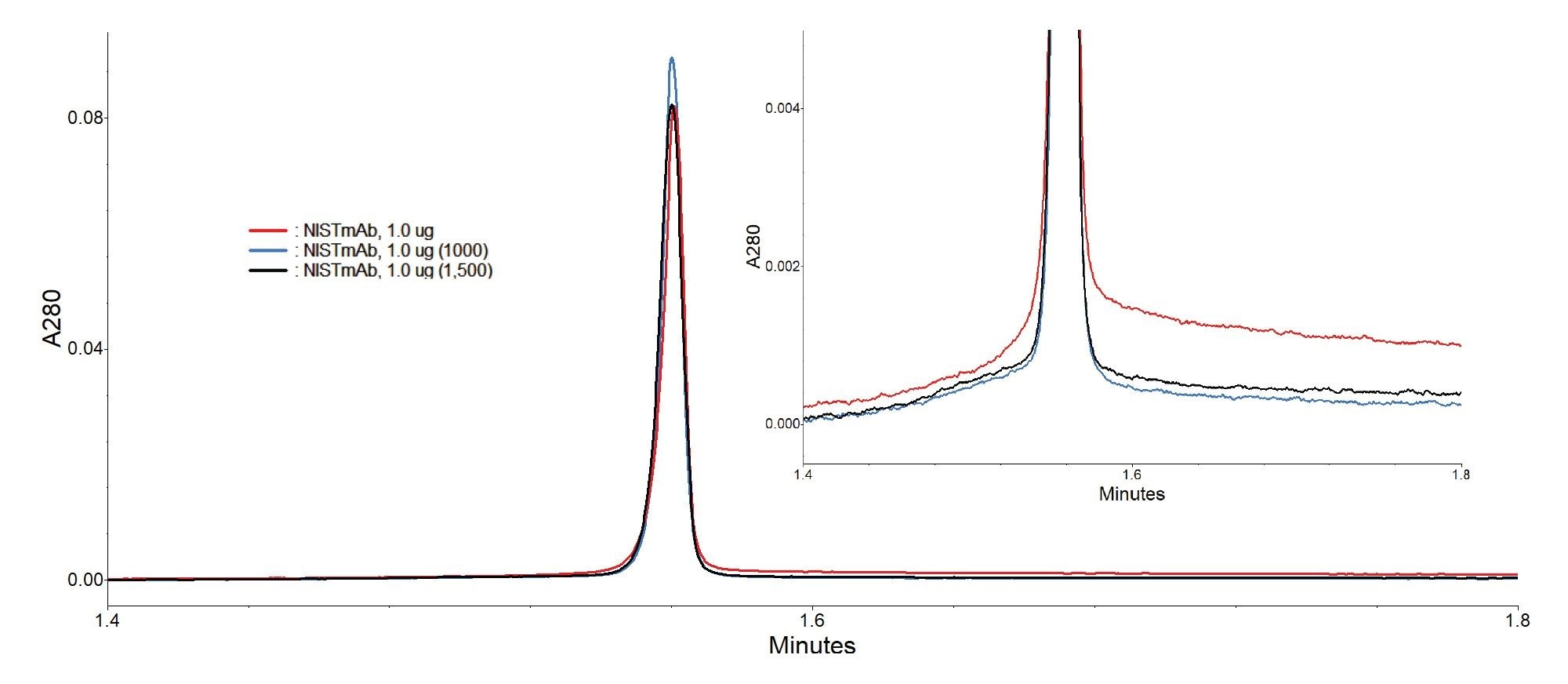
A similar approach to column lifetime analysis was then taken with the 3.9 x 5 mm column. For this configuration, the flow rate was increased to 2.0 mL/min to account for the linear velocity reduction due to the larger internal diameter as compared to the 2.1 x 20 mm column. Though this augmentation to flow rate did not result in identical linear velocities between both form factors, 2.0 mL/min is the maximum flow rate that this UPLC system can achieve and represents a relatively high flow rate for the intended application.
Benchmarking of the 3.9 x 5 mm column lifetime in the absence of a CIP protocol was initiated, following the same injection sequence as described for the 2.1 x 20 mm column: 200 injections of spiked media, followed by a calibration curve generated using NISTmAb in 2x PBS, with the 1 µg injection serving as the indicator of chromatographic performance. As with the 2.1 x 20 mm column, backpressure was monitored during all injections regardless of sample matrix. After completing 1,600 media injections without CIP, chromatographic performance remained consistent and column backpressure increased by only 4.8%. The decision was made to perform a clean-in-place method at that time and after every 500 subsequent media injections, with the goal of maximizing column lifetime through in-process column and system cleaning. The CIP injection protocol remained the same as for the 2.1 x 20 mm column, but at an elevated flow rate of 0.5 mL/min. With the implementation of CIP, the 3.9 x 5 mm column withstood 2,400 spiked media injections with a 21.2% increase in column backpressure. Recovery and dynamic binding capacity of this column remained stable throughout the duration of lifetime testing.
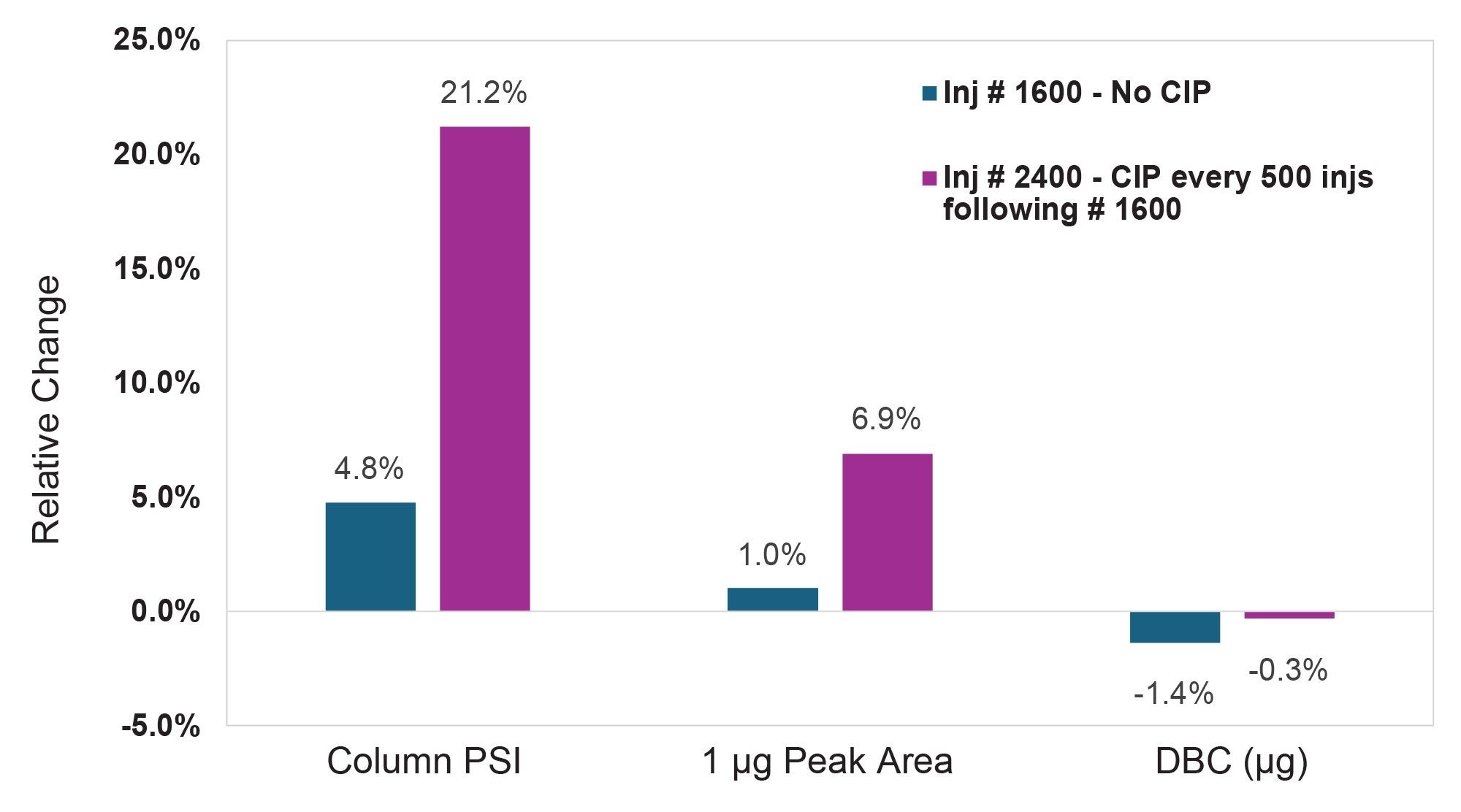
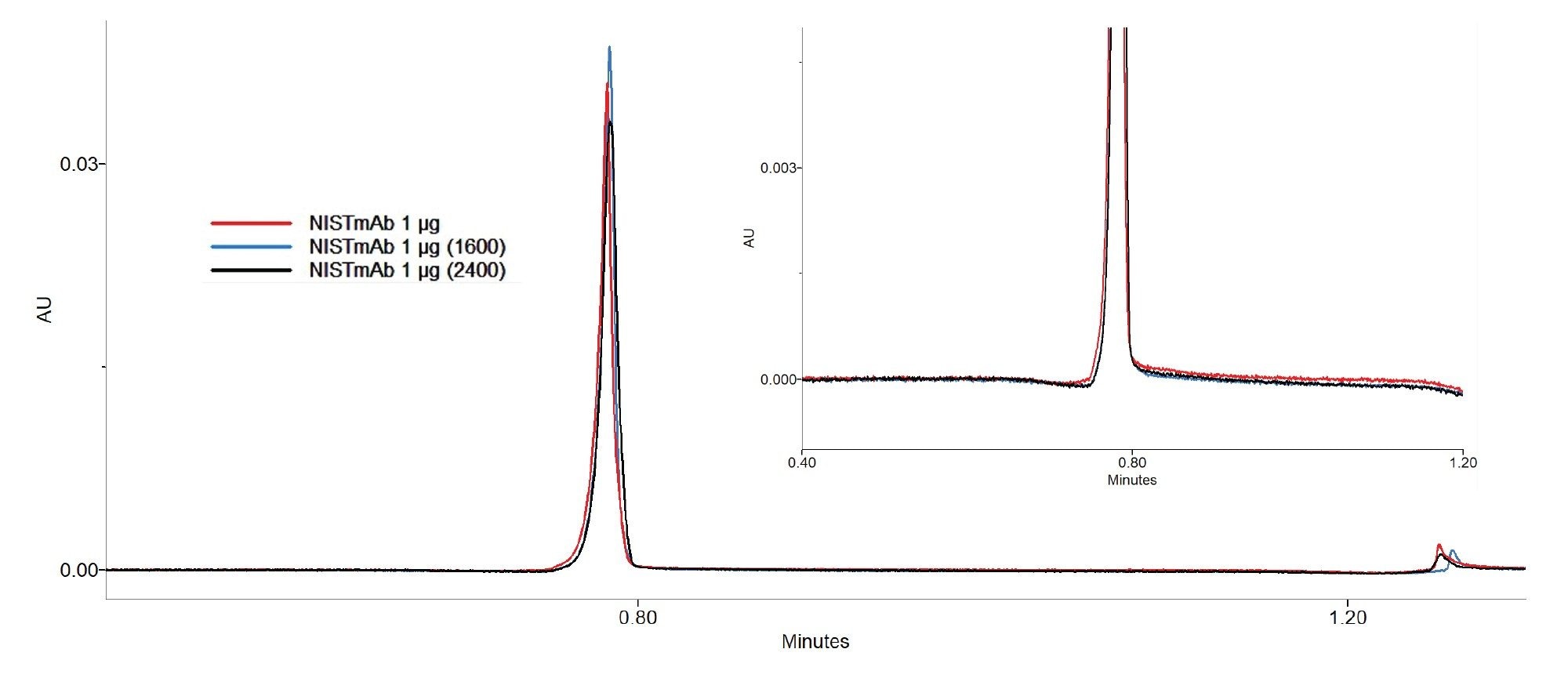
Conclusion
The testing performed in this study demonstrated that column lifetimes in excess of 1,500 injections can be achieved with Waters BioResolve Protein A Affinity Columns, though actual performance may vary based on the method conditions applied. Both configurations were packed with the same robust, high-performance protein A resin, however, the column dimensions had a measurable impact on the lifetime performance of the column. Therefore, it is essential for end users to select the column configuration that best aligns with the specific needs of their analytical workflows.
- The highly sensitive 2.1 x 20 mm column demonstrates excellent peak shape, albeit with the tradeoff of shorter column lifetime due to media fouling, which can be attenuated with a regularly implemented CIP procedure. Under these conditions, column longevity was demonstrably extended from 500 to 1,500 media injections following the introduction of a CIP protocol.
- The robust 3.9 x 5 mm column sacrifices some sensitivity as compared to the 2.1 x 20 mm column, but it can enable nearly twice the number of titer analysis injections, even with limited CIP. In this study, 1,600 media injections were achieved before any cleaning was performed, and the column ultimately reached 2,400 injections with just two CIP cycles.
- The rugged BioResolve Protein A Affinity resin withstood thousands of load and elute pH cycles and all CIP injections with minimal effect on recovery and column capacity.
References
- Pathak, M. and Rathore, A.S. Mechanistic understanding of fouling of protein A chromatography resin. Journal of Chromatography A, 1459, pp.78–88. 2016.
- Lintern, K., et al. Residual on Column Host Cell Protein Analysis during Lifetime Studies of Protein a Chromatography. Journal of Chromatography A, 1461, pp. 70–77. 2016.
- Hober, S., Nord, K. and Linhult, M. Protein a Chromatography for Antibody Purification. Journal of Chromatography B, 848(1), pp. 40–47. 2007.
- Paula Hong, Kenneth J. Fountain. Guidelines for Routine Use and Maintenance of Ultra-Performance Size-Exclusion and Ion-Exchange Chromatography Systems. Waters Application Note. 720004182, January 2012.
- Pamela C. Iraneta, Steven Byrd. Best Practices for Maintaining Column Performance in Size-Exclusion Chromatography during Long-Term Storage. Waters Application Note. 720007077, November 2020.
Featured Products
720009144, December 2025