Development of a Targeted Multiple Reaction Monitoring (MRM) Method for Fentalogues and Nitazene Derivatives for Use with Waters Xevo TQ-S micro Mass Spectrometer
aMatthias Mäder, aChristina Tanner, aIvan Schlatter, aDr. Stefan Nückel, bMarco Rentsch
aForensisch-Naturwissenschaftlicher Dienst, Kantonspolizei St. Gallen, Moosbruggstrasse 11, 9001 St. Gallen, Switzerland
bWaters AG, Täfernstrasse 14a, 5405 Baden-Dättwil
Published on December 12, 2025
Abstract
The emergence of highly potent synthetic opioids, including fentanyl and its analogues and nitazene derivatives, poses a growing challenge for forensic laboratories, particularly as diacetylmorphine (heroin) purity declines and low-level synthetic opioid adulteration becomes more common. Standard drug identification procedures, such as immunoassay, often fail to detect these compounds, many of which are not yet under international control. This work presents the development of a targeted multiple reaction monitoring (MRM) method for the identification of 250 fentanyl analogues often referred to as ‘fentalogues’ and nitazene derivatives, which also include metabolites, using Traceable Opioid Material® (TOM) Kits® (Cayman Chemical Ann Arbor, Michigan, USA Item Nos. 9003237, 9003286, 9003380, and 9003381). The method was developed using the ACQUITY™ UPLC™ I-Class System combined with the Xevo™ TQ-S micro Mass Spectrometer, together with the QuanOptimize Software platform to assist with the automated method development. This ready-to-implement solution provides optimized MRM transitions, retention times, and Product Ion Confirmation (PIC™) Spectra, together with pre-configured acquisition in MassLynx™ Software and processing methods in TargetLynx™ Application Manager, enabling the sensitive, selective, and confident screening and identification of synthetic opioids in both seized drug samples and biological matrices.
Benefits
- Ready-to-implement solution: The package with preconfigured methods and full reference data for 250 analytes is available on request from a Waters representative.
- Confidence in identification: Achieved through fixed chromatography with predefined retention times, up to four MRM transitions per analyte, and PIC spectra for direct comparison.
- Comprehensive Analysis: Target 250 fentalogues and nitazene derivatives, including their metabolites, providing characterization for both seized drug analysis and analysis of biological matrices.
Introduction
The global allocation of opium, particularly diacetylmorphine (heroin), can pose a challenge for drug enforcement agencies. Afghanistan was the major contributor for the production of crude opium; however, the Taliban implemented a strict ban on the cultivation of poppy in April 2022, leading to a sharp decline of heroin production.1,2 Although heroin stockpiled prior to 2022 appears to be sufficient to meet current demand, there is a clear downward trend in the quantity of the active ingredient in seized heroin samples. It is increasingly common that analyses by German or Swiss authorities have identified consignments containing as little as 2% heroin. These samples were found to be largely comprised of caffeine and paracetamol and, in certain instances, adulterated with low concentrations of opioids, such as counterfeit oxycodone and benzodiazepines.
Since 2018, the number of seizures of illicit fentanyl in North America has increased substantially, rising from 2,900 in 2018 to approximately 19,500 in 2023, representing a 6.7-fold increase.2 It was shown that some of the novel synthetic opioids can exhibit a strong analgesic potency, considered approximately 100 to 1000 times greater than morphine. This increase in popularity of fentanyl consumption, has led to approximately 74,000 overdose fatalities in the US during 2023.2 The European Union Drugs Agency (EUDA) reports an increased availability of these synthetic opioids as well as associated harms and deaths due to their high potency and novelty in Europe in the past years.1 Many of the fentalogues and nitazene derivatives are under international control but there are plenty of not listed derivatives available. The accurate identification of the various, highly potent synthetic opioids is a challenge for forensic laboratories as standard analytical procedures fail to detect them. As such, advanced techniques and screening protocols are required to address the monitoring of complex illicit drug formulations.
Tandem mass spectrometry has become a widely used technique in drug screening due to its high analytical sensitivity and selectivity in MRM mode. MRM can be applied for both quantitative analysis and targeted qualitative screening for which Waters has developed a comprehensive targeted databases over the years.4–6 This study describes the development of a new MRM method, specifically designed to identify 250 fentalogues and nitazene derivatives using the TOM Kits.3
The method was developed on the ACQUITY UPLC I-Class System and Xevo TQ-S micro Mass Spectrometer (Figure 1) using the chromatographic conditions previously established for the Waters MRM Toxicology Screening Methodology.4,5 The QuanOptimize Application Manager was used to automate MRM method development.6 This tool is particularly valuable for developing large compound methods, as it automatically optimizes the cone voltage for the precursor ion, identifies product ions, and optimizes the collision energies for each product ion through flow injections. Following this optimization step, a second analysis step can be programmed to inject the compounds onto the column for retention time determination.
This synthetic opioids MRM method includes the acquisition method in MassLynx Software, the processing method in TargetLynx Application Manager, and detailed documentation with retention times and PIC spectra for all 250 analytes.

Experimental
The ACQUITY UPLC I-Class System and Xevo TQ-S micro Mass Spectrometer was controlled by MassLynx Software v4.2. QuanOptimize was used for automatic MRM method development, while the data were processed using the TargetLynx XS Application Manager.
Test Substances
Fentanyl Analog Screening (FAS) kit (Item No. 9003237) and Emergent Panels version 1 (Item No. 9003286), Emergent Panels version 2 and 3 (Item No. 9003380) and Emergent Panels version 4 (Item No. 9003381) were obtained from Cayman Chemical. The kits are supplied in a 96‑well plate format with 700 µL vials, to which 700 µL of methanol was added, yielding a nominal concentration of 286 µg/mL.
Samples for QuanOptimize: A 35 µL aliquot of the test substance was diluted with 965 µL of methanol to obtain a concentration of 10 µg/mL. From this solution, 50 µL were further diluted with 950 µL of methanol to yield a final concentration of 500 ng/mL. An injection volume of 0.2 µL was used in flow injection mode with the column manager set to bypass.
Samples for analysis: A 20 µL aliquot of the samples prepared for QuanOptimize was diluted with 980 µL of methanol to obtain a concentration of 10 ng/mL. Subsequently, 100 µL of a propranolol internal standard stock solution (87 ng/mL) were added to 1000 µL of this solution, resulting in a final internal standard concentration of 7.9 ng/mL. Finally, 1 µL of the mixture was injected under the conditions described below.
LC Conditions
Mobile Phase A (5mM Ammonium Formate, pH 3.0)
To prepare the buffer, approximately 900 mL of Milli-Q® (Merck KGaA) water (18.2 MΩ at 25 °C, <5 ppb TOC) or LC‑MS‑grade water was transferred into a 1 L volumetric flask. Then, 1 mL of solution 186007361–2 (ammonium formate buffer 500 mM, 9.92% formic acid) from the Waters Forensic Toxicology Installation Standards Kit was accurately added. The volumetric flask was filled to volume with Milli‑Q or LC‑MS‑grade water, capped, and mixed thoroughly by shaking or sonicating. The prepared buffer was transferred into a bottle suitable for LC‑MS.
Mobile Phase B (Acetonitrile 0.1% Formic Acid)
A total of 1,000 mL of LC‑MS‑grade acetonitrile was accurately measured in a graduated cylinder or volumetric flask and transferred into a bottle suitable for LC‑MS. Then, 1.0 mL of LC‑MS‑grade formic acid was accurately added. The solution was mixed thoroughly, and the bottle was placed on the ACQUITY UPLC I-Class System.
Column
Waters ACQUITY UPLC HSS C18 Column, 2.1 x 150mm, 1.8 µm (p/n: 186003534) at 50 °C.
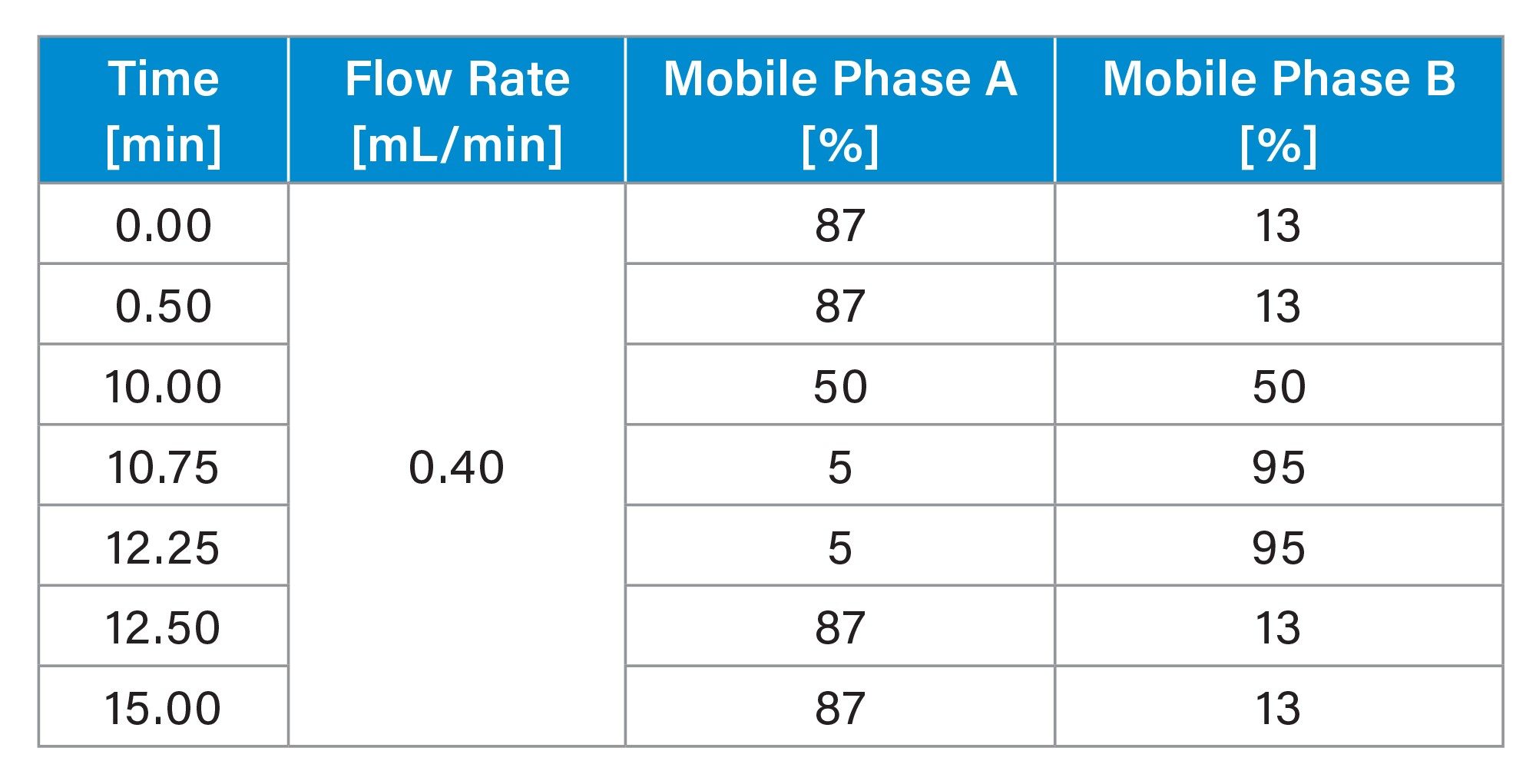
Gradient Timetable
MS Conditions
|
MS system: |
Xevo TQ-S micro Mass Spectrometer |
|
Ionization mode: |
Electrospray ionization in positive mode |
|
Acquisition mode: |
MRM with PIC Scan |
|
Capillary voltage: |
1.0 kV |
|
Source temperature: |
150 °C |
|
Desolvation temperature: |
400 °C |
|
Desolvation gas flow: |
800 L/Hr |
|
Cone: |
20 L/Hr |
MRM Parameters
Detection and quantification were achieved through fixed chromatography with predefined retention times and up to four MRM transitions per analyte. PIC spectra were acquired for direct comparison.
Results and Discussion
Using QuanOptimize, a targeted library for 250 fentalogues and nitazene derivatives was rapidly developed, consisting of up to four MRM transitions per compound to provide confidence in the identification between closely related derivatives. Additionally, PIC spectra were recorded. PIC is an acquisition mode in which the intensity of an MRM channel triggers the acquisition of a corresponding product ion spectrum, enabling a reference product ion spectrum to be stored for each compound in the library. During data processing with the TargetLynx Application Manager, the product ion spectra, which were acquired during the sample analysis, were automatically compared with the reference spectra, calculating a match score to confirm analyte identity.
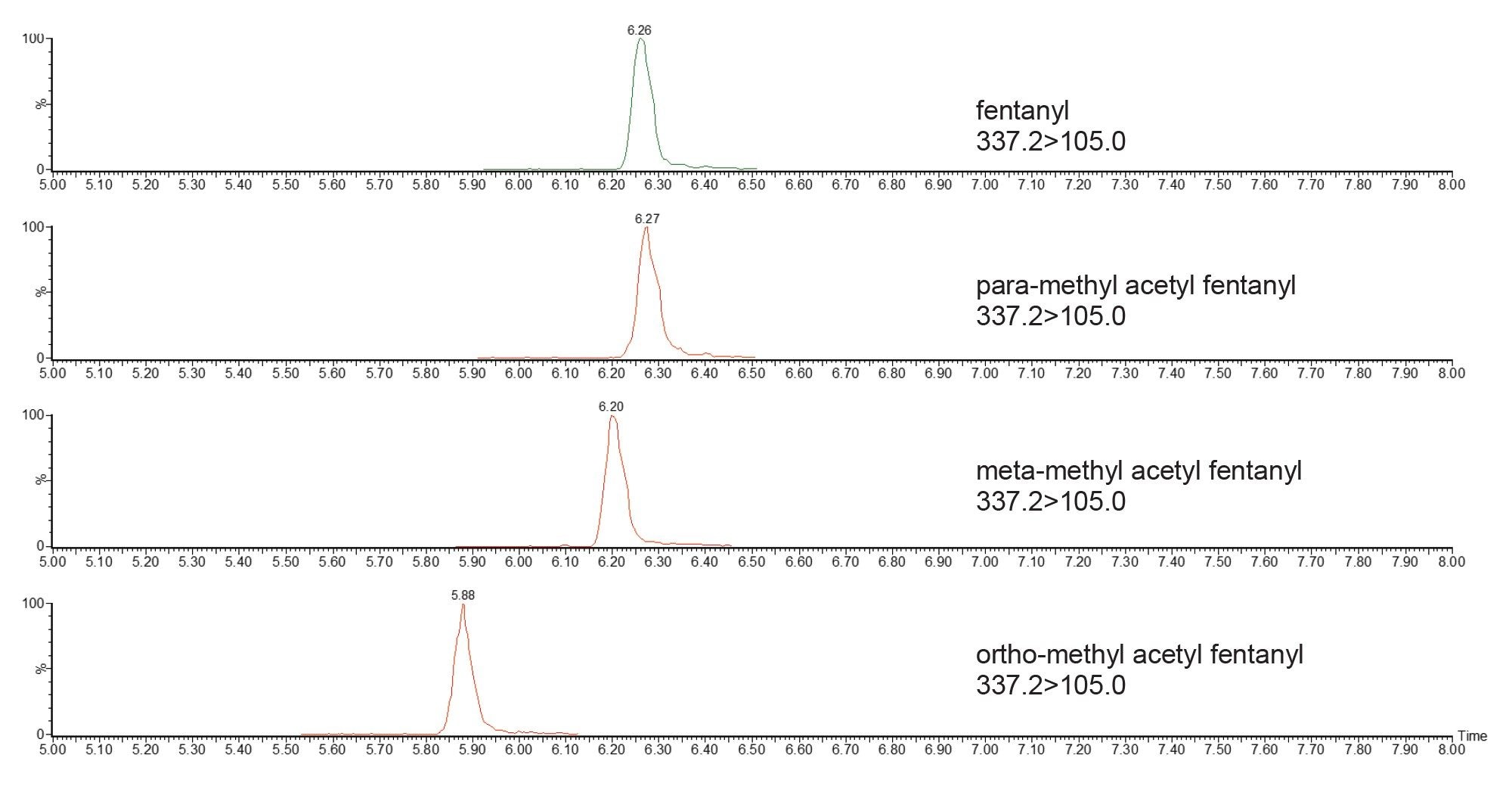
The fragmentation mechanisms of fentanyl and its analogues have been extensively characterized, with the fragmentation occurring at the N-4C and N-αC bonds (Figure 2), creating the product ions m/z 188.1 and m/z 105.1, respectively.7 This was confirmed by the PIC spectrum for fentanyl (Figure 3). This method monitors ten fentanyl isomers. The ortho-, meta-, and para-isomers cannot be distinguished by their product ion spectra, due to their identical fragmentation; m/z 188.1 and m/z 105.1. Chromatographic separation was achieved for ortho-methyl acetyl fentanyl; however, the meta- and para- isomers elute closely with fentanyl (Figure 4).
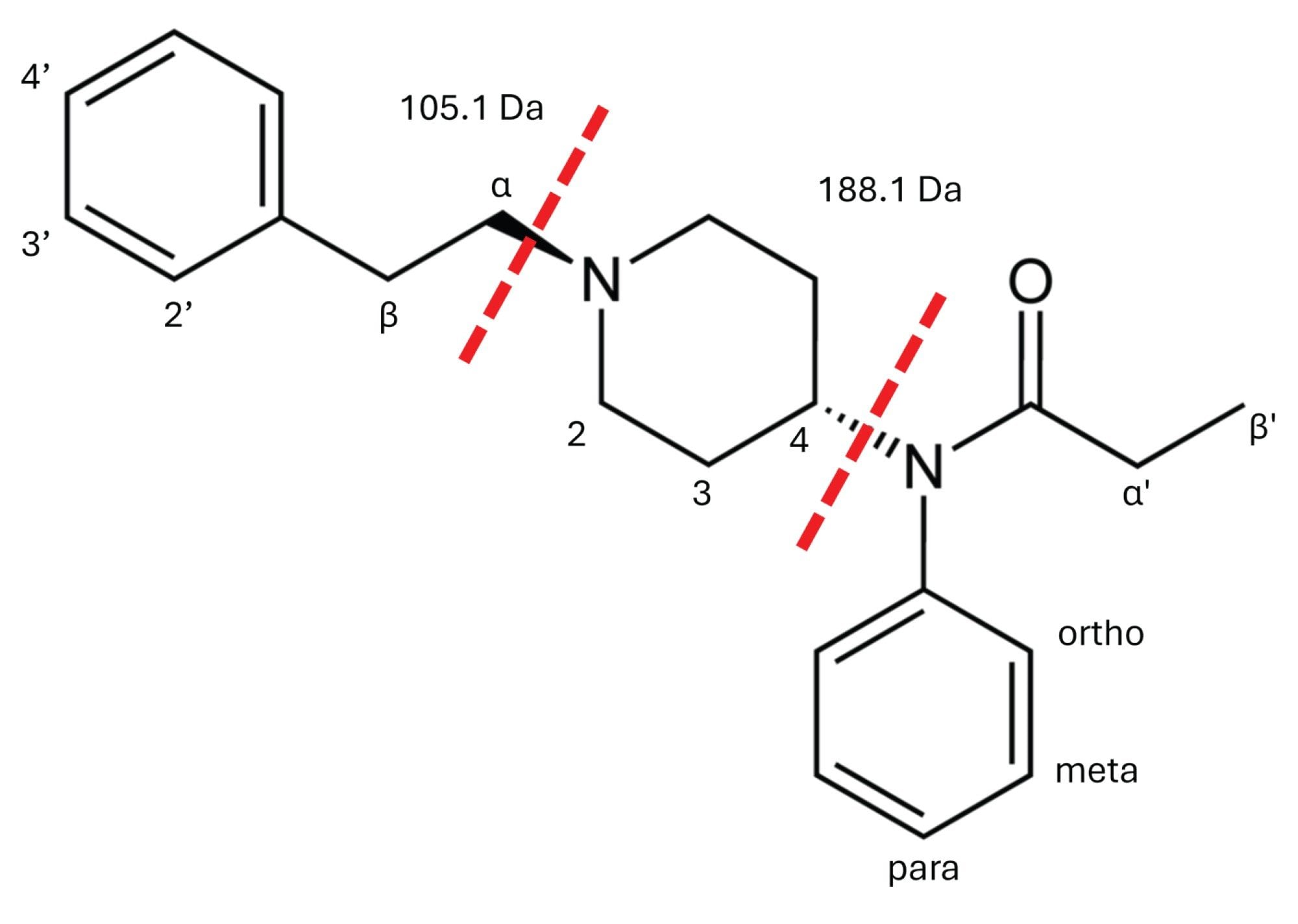
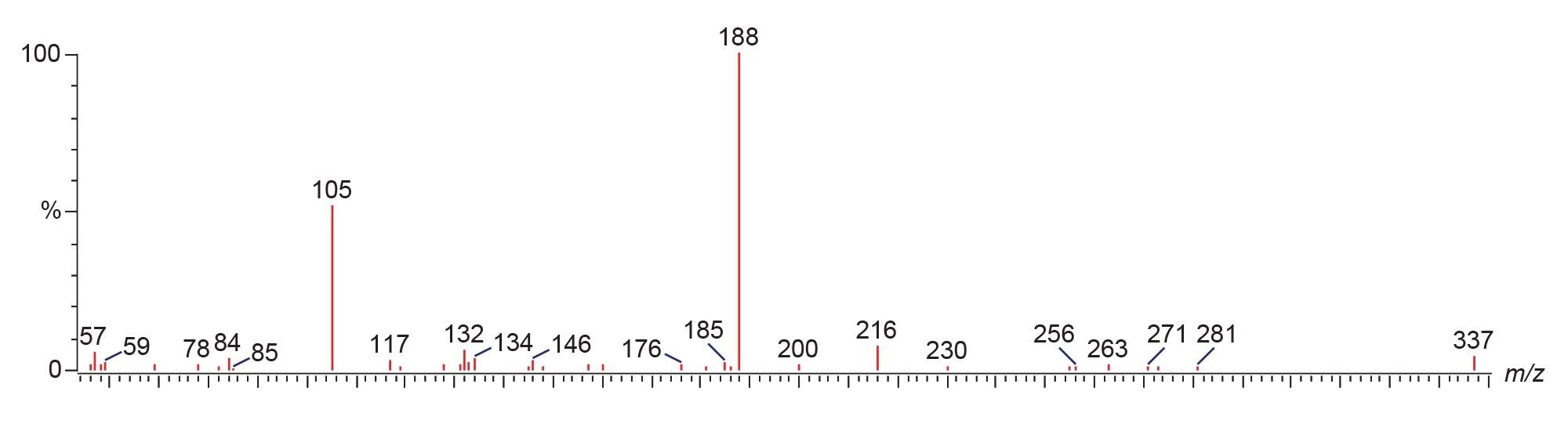
For fentanyl analogues, structural modification at positions α, β, 2’, 3’, 4’ (R1) result in a fragment mass of m/z 105.1 + R1. Additional modifications at positions 2, 3, and 4 (R2) produce a fragment of m/z 188.1 + R1 + R2. For example, β-methyl acetyl fentanyl shows PIC spectrum fragments at m/z 202.2 and m/z 119.1 (Figure 5), confirming the presence of a methyl group in R1.
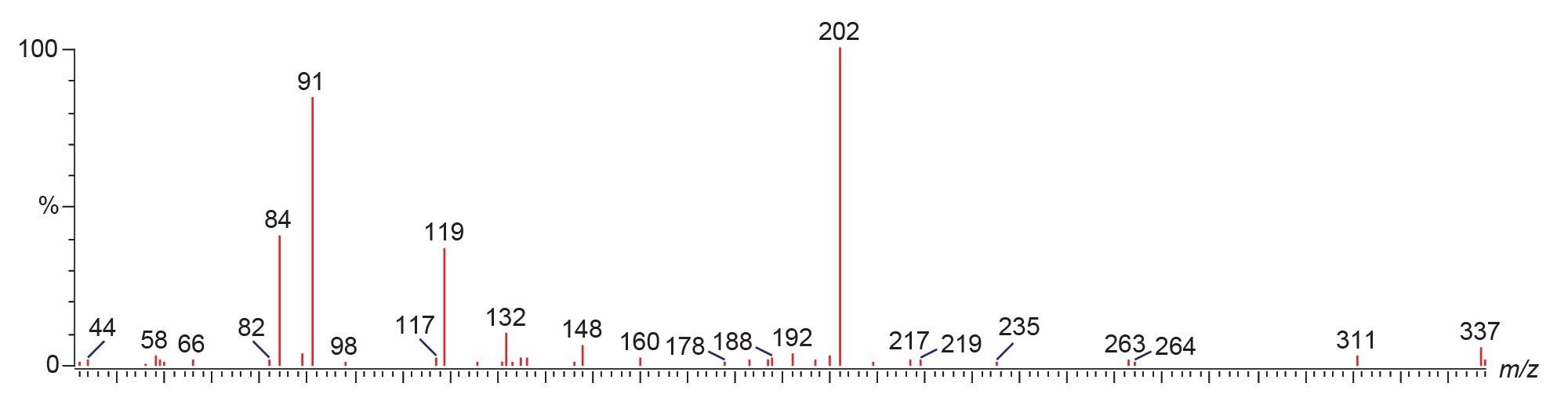
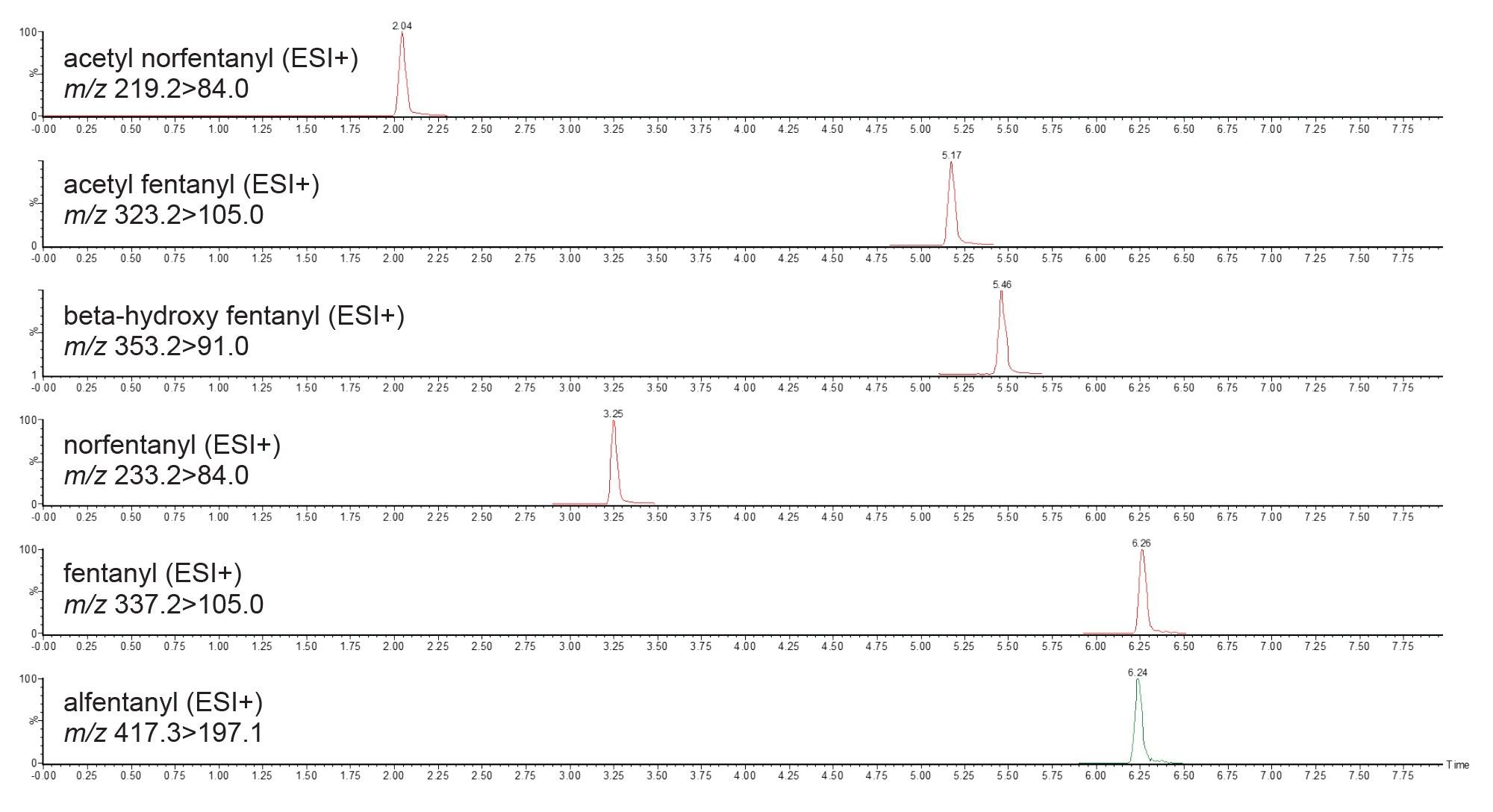
The method also comprises MRM transitions and PIC spectra for metabolites of fentanyl analogues. Figure 6 presents an example of the fentanyl analogues and their metabolites that are monitored.
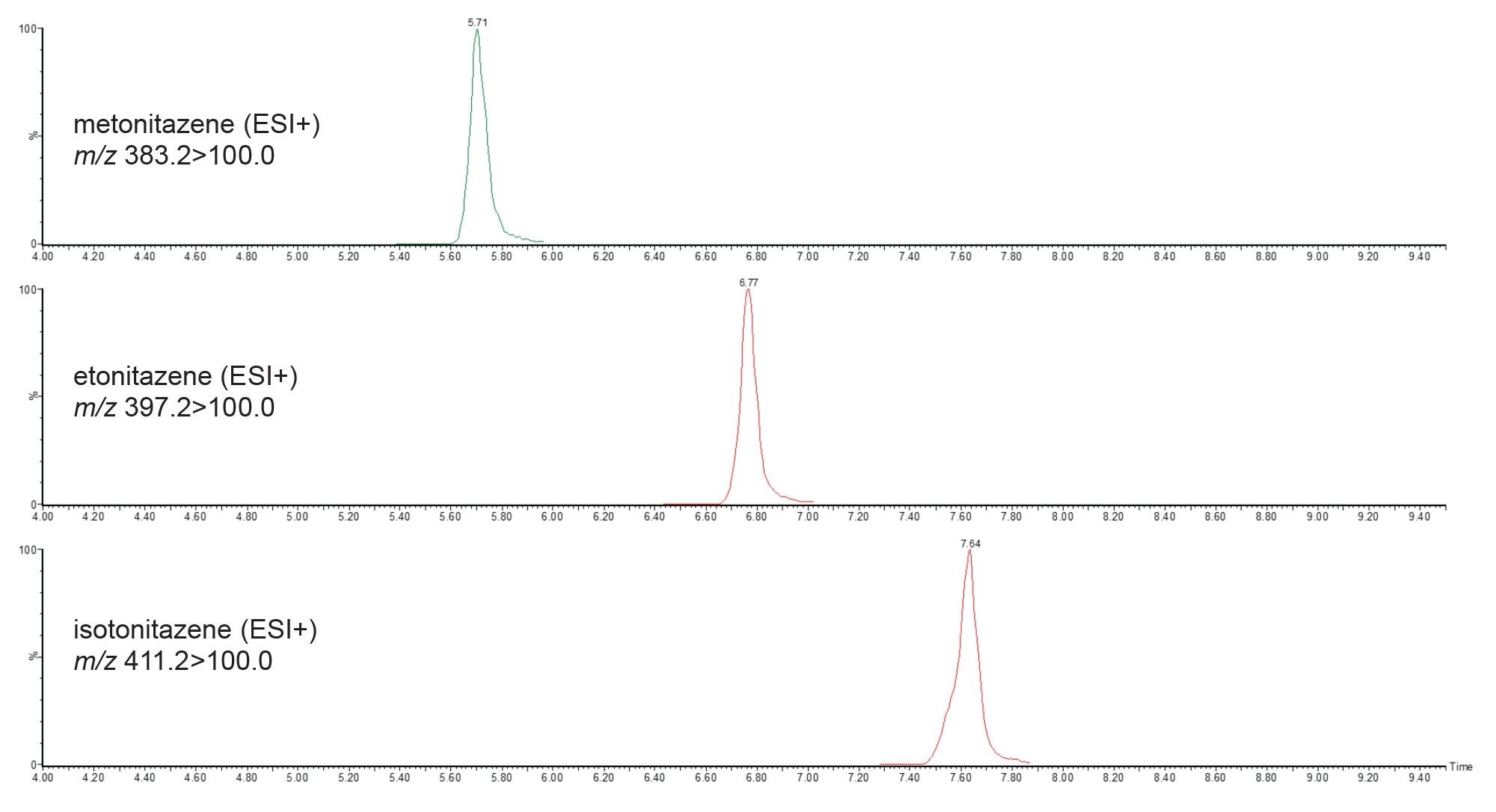
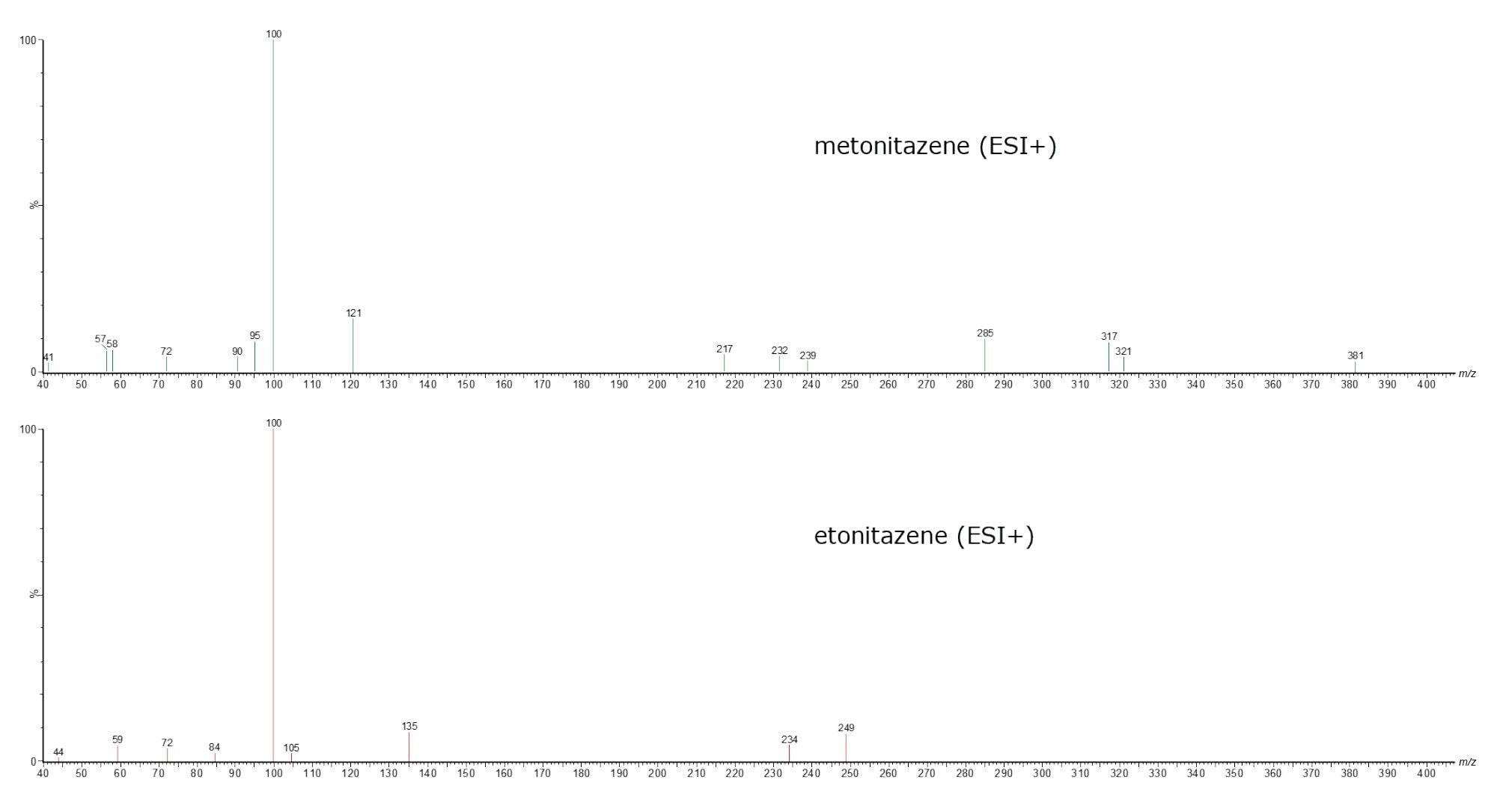
The method also includes three nitazene derivatives, each with its respective PIC spectrum. Figure 7 illustrates the separation of these derivatives. Etonitazene produces characteristic product ions at m/z 135.0, 100.0, 72.0, and 44.0; however, metonitazene, having a methoxy substitution, produces a characteristic fragment at m/z 121.0 (Figure 8).
Conclusion
This specifically developed targeted fentalogues and nitazene MRM method, provides a robust, sensitive, and ready-to-implement solution for forensic and toxicology laboratories. By utilizing up to four MRM transitions, combined with reference retention time and PIC spectra comparison, laboratories can report the detection and identification of synthetic opioids with confidence. Its seamless compatibility with the already established Waters toxicology screening method enables rapid integration into existing protocols, allowing laboratories to respond promptly and effectively to the evolving synthetic opioid crisis.
Not for use in clinical toxicology procedures.
Acknowledgment
Laboratory findings were made possible, in part, by the Centers for Disease Control and Prevention’s design and support of Traceable Opioid Material Kits. The Fentanyl Analog Screening (FAS) kits were generously provided free of charge by Cayman Chemical, Ann Arbor, Michigan, USA.
Waters, ACQUITY, UPLC, Xevo, PIC, MassLynx and TargetLynx are trademarks of Waters Technologies Corporation. Traceable Opioid Material is a trademark of U.S. Department of Health and Human Services. Milli-Q is a trademark of Merck KGaA.
References
- European Union Drugs Agency. Heroin and other opioids – the current situation in Europe (European Drug Report 2025), published online. https://www.euda.europa.eu/publications/european-drug-report/2025/heroin-and-other-opioids_en.
- https://www.unodc.org/unodc/en/data-and-analysis/wdr-drug-market-patterns-trends.html.
- Cayman Chemical Company, Ann Arbor, MI; Fentanyl Analog Screening (FAS) kit and Emergent Panels version 1 to 4, released between January 2019 and February 2021; contains 250 synthetic opioid compounds, supplied at stock solutions at 1 mg/mL.
- Lee, Robert, Wood, Michelle. Targeted MRM Screening Using the ACQUITY UPLC I-Class/ Xevo TQ-S micro. Waters Application Note. 720005606. February, 2016.
- Mistry, Nayan S., Cooper, Jane, Wood, Michelle. Expansion of the MRM Toxicology Screening Methodology for Use With Waters Xevo TQ-S micro. Waters Application Note. 720007748. October, 2022.
- Tanna, Nikunj. QuanOptimize: A Software Tool that Enables Rapid, Consistent, and Accurate MRM Method Development for Large Numbers of Small Molecule Analytes. Waters Application Note. 720007002. September, 2020.
- Yu Zhang, John C. Halifax, Christina Tangsombatvisit, Cassandra Yun, Shaokun Pang, Shirin Hooshfar, Alan H.B. Wu, Kara L. Lynch. Development and application of a High-Resolution mass spectrometry method for the detection of fentanyl analogs in urine and serum, Journal of Mass Spectrometry and Advances in the Clinical Lab, Volume 26, 2022, Pages 1–6, https://doi.org/10.1016/j.jmsacl.2022.07.005.
720009165, December 2025