Verifying Performance and Compliance of a Gel Permeation Chromatography (GPC) Method With the Requirements in a Proposed USP Monograph for Sorbitan Monostearate
Abstract
The proposed United States Pharmacopeia (USP) monograph for sorbitan monostearate utilizes a GPC method with refractive index (RI) detector for the assay and a limit test of organic impurities (USP-PF 50(2)). This application note demonstrates performance and implementation of a proposed USP monograph for sorbitan monostearate on an Arc HPLC™ System with a strong solvent compatibility kit and RI detector. Results generated by the method met all the USP requirements for system suitability and acceptance criteria for the assay and organic impurities testing in sorbitan monostearate.
Benefits
- Successful performance verification of a proposed USP monograph for sorbitan monostearate by demonstrating compliance with all the requirements
- Excellent GPC results generated on the Arc HPLC System with a strong solvent compatibility kit and RI detector, meeting the USP specifications for system suitability and acceptance criteria for the assay and organic impurities testing
Introduction
Sorbitan monostearate (Span 60), is a non-ionic surfactant with emulsifying, dispersing, and wetting properties used in the production of food and and health care products.1–2 It is primary used as an emusifier to prevent ingredients from separating in many food products such as margarine, chocolate cream, and similar dairy products.2 In yeast manufacturing, it protect the yeast from excess drying and also helps rehydrate the yeast cells.1 Sorbitan monostearate is a synthetic ester made from sorbitol and stearic acid.
The USP has launched an initiative to update and improve an outdated monographs for drug substances, drug products and excipients published in the United States Pharmacopeia—National Formulary (USP–NF) compedia.3–4 As part of the moderniation effort, the USP proposed revisions to the monograph for sorbitan monostearate to update assay procedure and add a limit of organic impurities testing.5 The assay procedure is designed to replace current tests for fatty acids and polyols testing with a GPC method for analysis of sorbitan tri-/higher esters, sorbitan diesters, and sorbitan monoester. The limit of organic impurities procedure operates under the same GPC chromatographic conditions as the assay.
In this work, a GPC method outlined in the proposed USP monograph for sorbitan monostearate was run an Arc HPLC with a strong solvent compatibility kit and RI detector. To demonstrate compliance with the proposed monograph, the results were compared against all requirements for system suitability and acceptance criteria for both the assay and organic impurities testing. Performance verification of the USP proposed monographs for sorbitan monooleate and sorbitan sesquioleate is demonstrated in a previously published application notes.6,7
Experimental
Solutions preparation and experimental conditions proceeded as described in the proposed USP monograph for sorbitan monostearate.5
Materials
Tetrahydrofuran (THF) HPLC grade, no preservatives, purchased from Fisher Chemicals, Catalog No: T425–4. Isopropyl alcohol (IPA) purchased from Honeywell, catalog number LC323–4. Sorbitan monostearate purchased form Sigma-Aldrich.
Sample Description
Standard solutions
Standard solution for assay prepared by dissolving each of stearic acid, 1,4-sorbitan, and isosorbide in tetrahydrofuran at 1.0 mg/mL. For limit of organic impurities, proceeded as described in the assay.
Sample Solutions
Sample solution for assay testing prepared by dissolving sorbitan monostearate in tetrahydrofuran at 1.0 mg/mL. For limit of organic impurities, proceeded as described in the assay.
Method Conditions
|
System: |
Arc HPLC System with quaternary solvent manager (QSM), flow through needle (FTN) sample manager, and strong solvent compatibility kit (p/n: 205002572). Column heater/cooler (p/n: 186179100) |
|
Detector: |
Refractive Index (RI) • Flow cell temperature: 30° C • Sampling rate: 10 pts/sec • Polarity: positive |
|
Mobile phase: |
Tetrahydrofuran |
|
Separation: |
Isocratic |
|
Columns: |
Columns with 7.8 x 300 mm with 5 µm, connected in series, starting with larger pore size using a joining tube (p/n: WAT084080) supplied with columns. 1. Styragel™ HR 1, 100 Å, molecular weight range: 100–5,000 (p/n: WAT044234) 2. Styragel HR 0.5, 50 Å, molecular weight range: 0–1,000 (p/n: WAT044231) |
|
Column temperature: |
30° C |
|
Sample temperature: |
25° C |
|
Flow rate: |
0.9 mL/min |
|
Injection volume: |
20 µL |
|
Run time: |
30 minutes |
|
Vials: |
LCMS Maximum Recovery 2 mL volume (p/n: 600000670CV) |
|
Wash solvents: |
Sample manager/purge wash: tetrahydrofuran Seal wash: isopropyl alcohol |
Assay and limit of organic impurities procedures operated under the same chromatographic conditions.
Data Management
|
Chromatography software: |
Empower™ 3 Feature Release 5 Service Release 5 (FR5 SR3) for data acquisition and analysis. |
Results and Discussion
The assay and limit for organic impurities procedures outlined in the proposed USP monograph for sorbitan monostearate utilize the same GPC separation conditions.5 The USP recommends Waters Styragel HR 0.5 and HR 1 columns with THF as a mobile phase delivered isocratically. Additionally, preparation of standard, and sample solutions for organic impurities is the same as that for the assay.
Peak Assignment
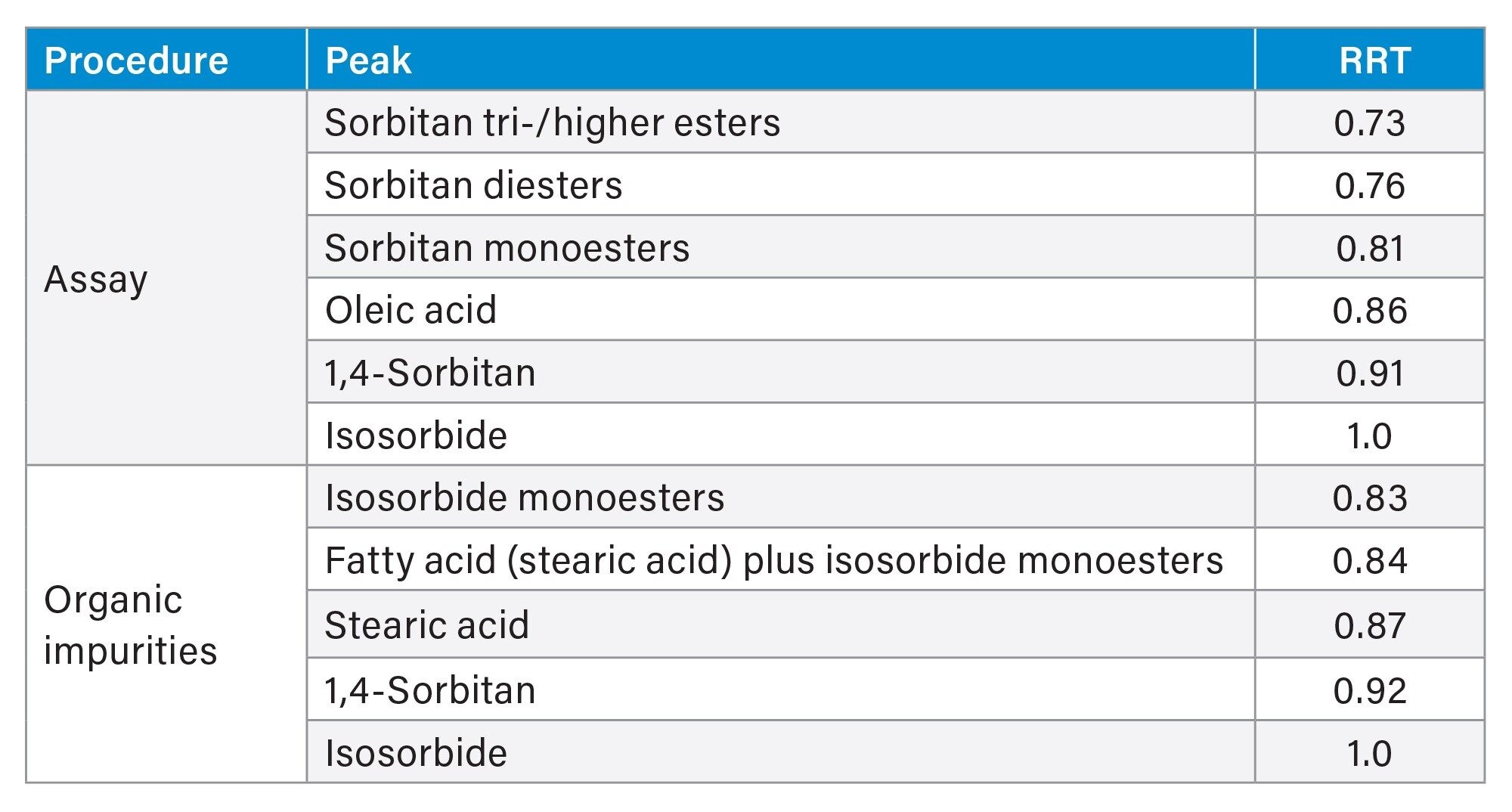
The identification of the sample components for the assay and organic impurities testing was performed according to the USP recommendation of relative retention times to aid peak assignment (Table 1).
System Suitability
System suitability results generated by the method were examined against the USP requirements for relative standard deviation (RSD) and resolution as specified in the proposed USP monograph for sorbitan monostearate.5 Solutions for system suitability determination include:
- Assay: standard and sorbitan monostearate sample solutions
- Limit of organic impurities: standard solution
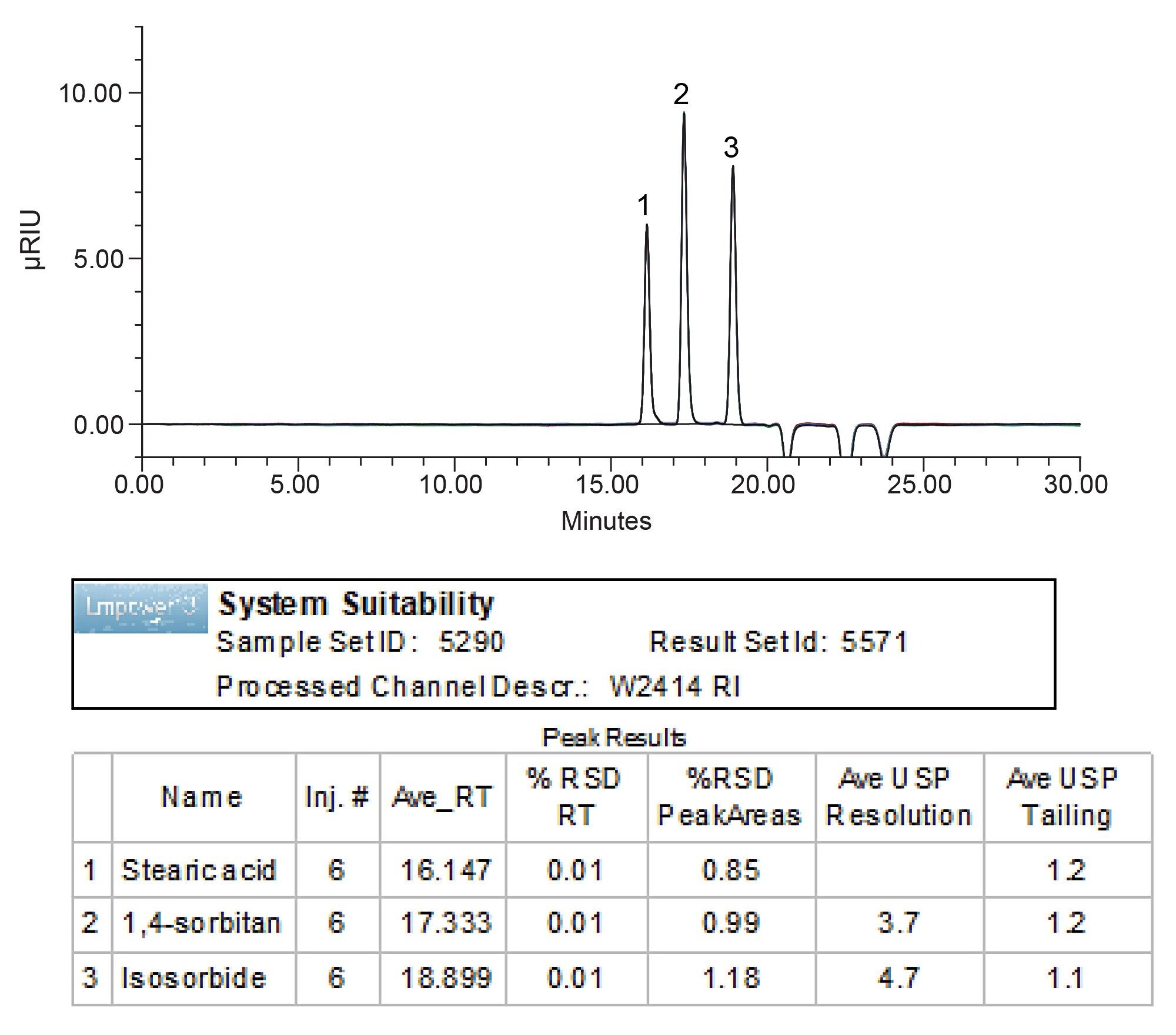
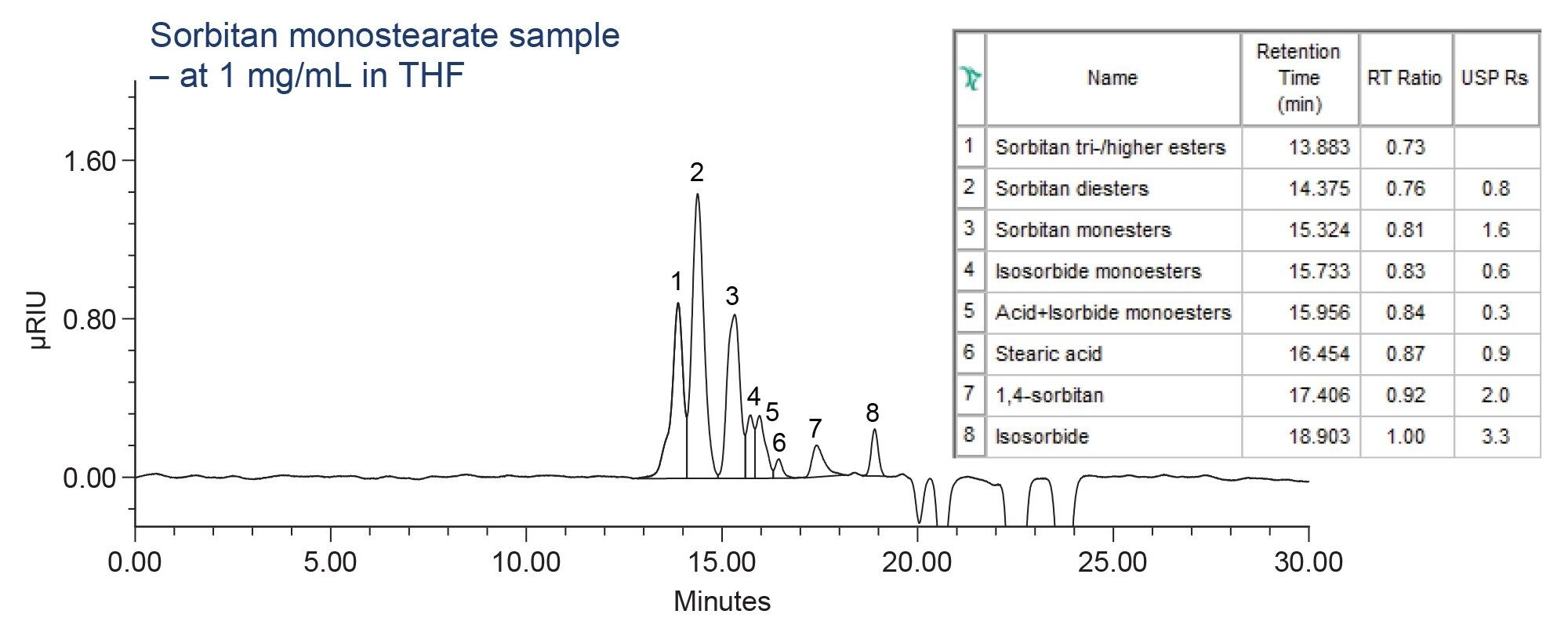
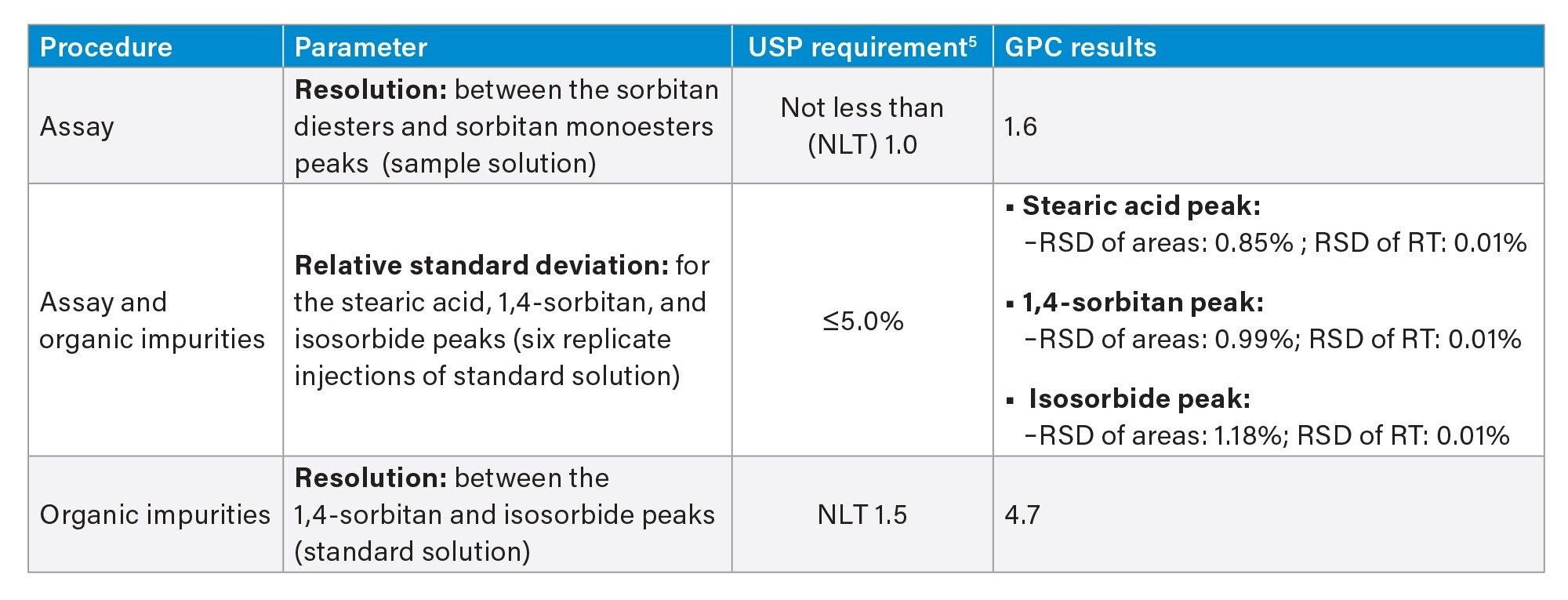
The relative standard deviation (RSD) for assay and organic impurities was assessed using six replicate injections of standard solution. Method produced excellent RSD for peak areas and retention times of ≤1.18% and ≤0.01%, respectively (Figure 1). Resolution for assay measured using sample solution was found to be 1.6, which is above the USP requirement of not less than (NLT) 1.0 (Figure 2). Method also met the resolution requirement for organic impurities of NLT 1.5 between the 1,4-sorbitan and, isosorbide peaks, producing value of 4.7 (Figure 1).
Overall, the GPC method on the Arc HPLC System successful met all the USP requirements specified for both procedures, summarized in Table 2.
Sorbitol Monostearate Sample Analysis
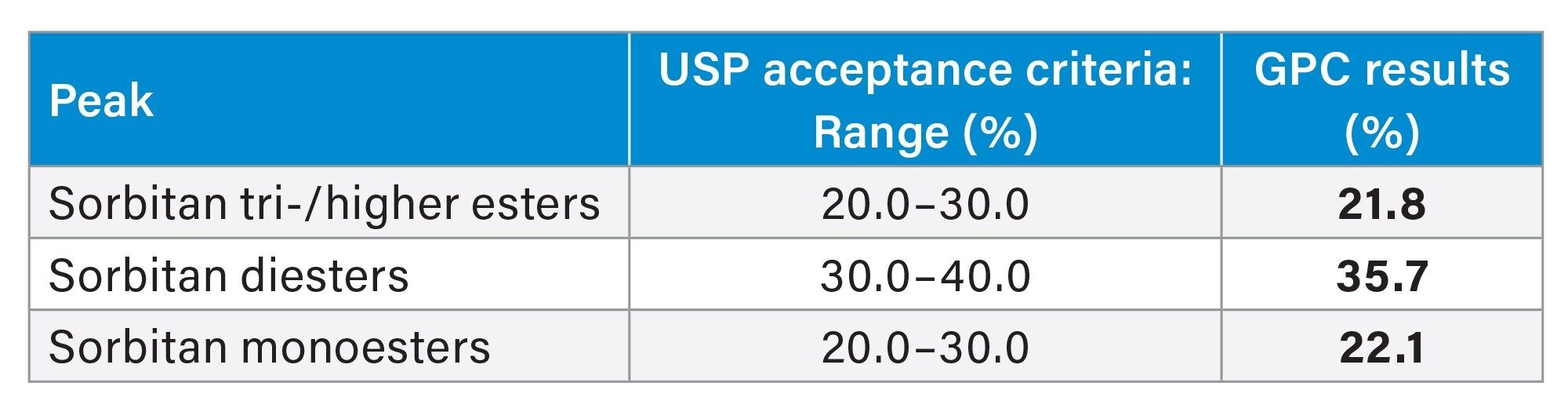
Assay: analysis of sorbitan tri-/higher esters, sorbitan diesters, and sorbitan monoesters
The percentage (%) of each sorbitan ester component in the sorbitan monostearate sample was calculated by area normalization as instructed by the USP.5 Area of individual peak was divided by the sum of the relevant peak areas and multiplied by 100. The assay results were within the USP acceptance criteria ranges (Table 3).
Limit of Organic Impurities
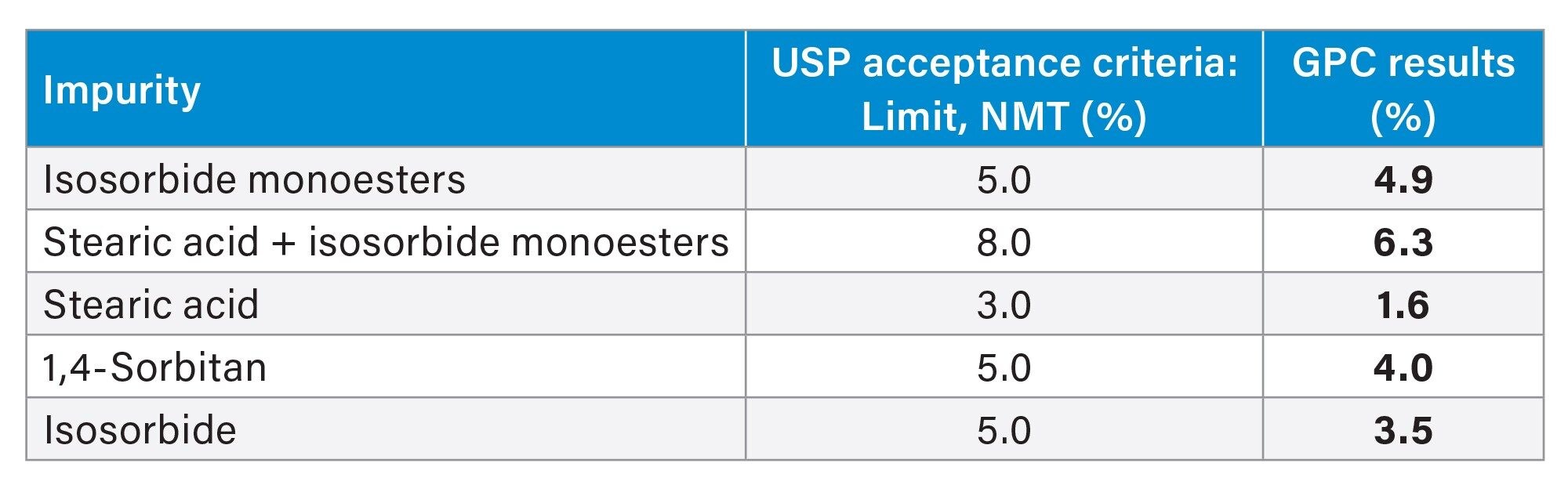
To determine the percentage (%) of each impurity in the sorbitan monostearate sample, the area of each individua peak was compared to the sum of the relevant peaks. The GPC results met the USP limits for organic impurities content (Table 4).
Conclusion
Excellent performance of the GPC method outlined in the proposed USP monograph for sorbitan monostearate was achieved on the Arc HPLC System with a strong solvent compatibility kit and RI detector. The system suitability results met all the USP specification for resolution and RSD of replicate injections. The results for assay and limit of organic impurities in the sorbitan monostearate sample met the acceptance criteria for percentages of relevant components. The GPC system delivered reliable, and reproducible, demonstrating compliance with the requirements and successful implementation of the USP monograph.
References
- https://www.omri.org/sorbitan-monostearate.
- https://www.echemi.com/cms/470675.html.
- United States Pharmacopeia and National Formulary (USP-NF), https://www.uspnf.com/notices/retired-compendial-notices/usp-monograph-modernization-initiative.
- https://www.americanpharmaceuticalreview.com/Featured-Articles/173161-USP-Monograph-Modernization-Initiative/.
- United States Pharmacopeia—National Formulary/ Pharmacopeial Forum (USP–NF) revision abstract for sorbitan monostearate, USP 50(2). 2024. https://doi.usp.org/USPNF/USPNF_M77610_30101_01.html.
- Maziarz M. Performance Verification of a Proposed USP Monograph for Sorbitan Monooleate Using a Gel Permeation Chromatography (GPC) Method With Refractive Index (RI) Detection. Waters Application Note. 720008502. 2024.
- Maziarz M. Demonstrating Compliance of a Gel Permeation Chromatography (GPC) Method With the Requirements in a Proposed USP Monograph for Sorbitan Sesquioleate. Waters Application Note. 720008613. 2024.
720008655, December 2024