Rapid Mixed-Mode SPE Method Development of Tyrosine Kinase Inhibitor Oncology Pharmaceuticals Oasis™ Method Development Plate
Abstract
The following work demonstrates the fast mixed-mode SPE screening approach for bioanalytical sample preparation, achieving high recovery and selectivity of several block-buster small molecule oncology therapeutic drugs from plasma using the Oasis Method Development µElution™ Method Development plate, containing four mixed-mode Oasis SPE sorbents for rapid extraction method development. Linear and accurate bioanalytical quantitation from plasma was achieved using MCX SPE extraction and subsequent LC-MS/MS analysis.
Benefits
- Oasis µElution Method Development Plate, containing three rows each of MCX, WAX, WCX, and MAX mixed-mode Oasis SPE sorbents for fast mixed-mode SPE analyte method development
- High analyte recovery (≥70%) and specificity (matrix effects ≤15%) using MM Oasis Sorbents, which includes both reversed-phase and ion-exchange functionality for orthogonal separation and retention
- Fast, three minute UPLC-MS analysis using the ACQUITY™ I-Class Plus UPLC™, ACQUITY UPLC CSH™ C18 Column, and Xevo™ TQ-XS Mass Spectrometer
- Robust quantitative performance achieving linearity and accuracy of TKI oncology pharmaceuticals from plasma using MCX SPE extraction
Introduction
Bioanalytical LC-MS methods are essential for the accurate quantitation of next-generation therapies and generic equivalents in biological matrices. Developing these methods requires robust and efficient sample preparation strategies to ensure highly accurate, sensitive, and selective methods. Sample preparation plays a critical role in bioanalytical workflows, significantly impacting method complexity and development time, but drives the ultimate successful implementation of a method.
While it affords many benefits over other extraction techniques like protein precipitation or traditional reversed-phase solid phase extraction (SPE), mixed-mode (MM) SPE can be perceived as complex, often requiring lengthy protocol optimization to achieve high recovery and reproducibility required for analyte quantification from biological matrices. However, mixed-mode SPE provides enhanced selectivity over other techniques and is a good alternative if reversed-phase SPE is not providing adequate recovery or selectivity. In this application, the Oasis µElution Method Development Plate, containing three rows each of MCX, WAX, WCX, and MAX mixed-mode Oasis SPE sorbents, was used for preliminary MM SPE extraction screening of several block-buster oncology tyrosine kinase inhibitors (TKI) pharmaceuticals (Table 1) from plasma.1 Excellent SPE recovery (≥70%) and low matrix effects (≤15%) was achieved using the MCX sorbent. Use of this method development plate with optimized protocols, enabled simple and rapid mixed-mode SPE screening in one experiment, which significantly reduced sample preparation method development time.
Final sample preparation and extraction of the TKI oncology pharmaceuticals from plasma was performed using the MM MCX 96-well µElution Plate and subsequent LC-MS/MS analysis. Linear and accurate bioanalytical quantification results were achieved for all analytes, with calibration curves ≥0.99 and accuracy ≥85% across the calibration points.
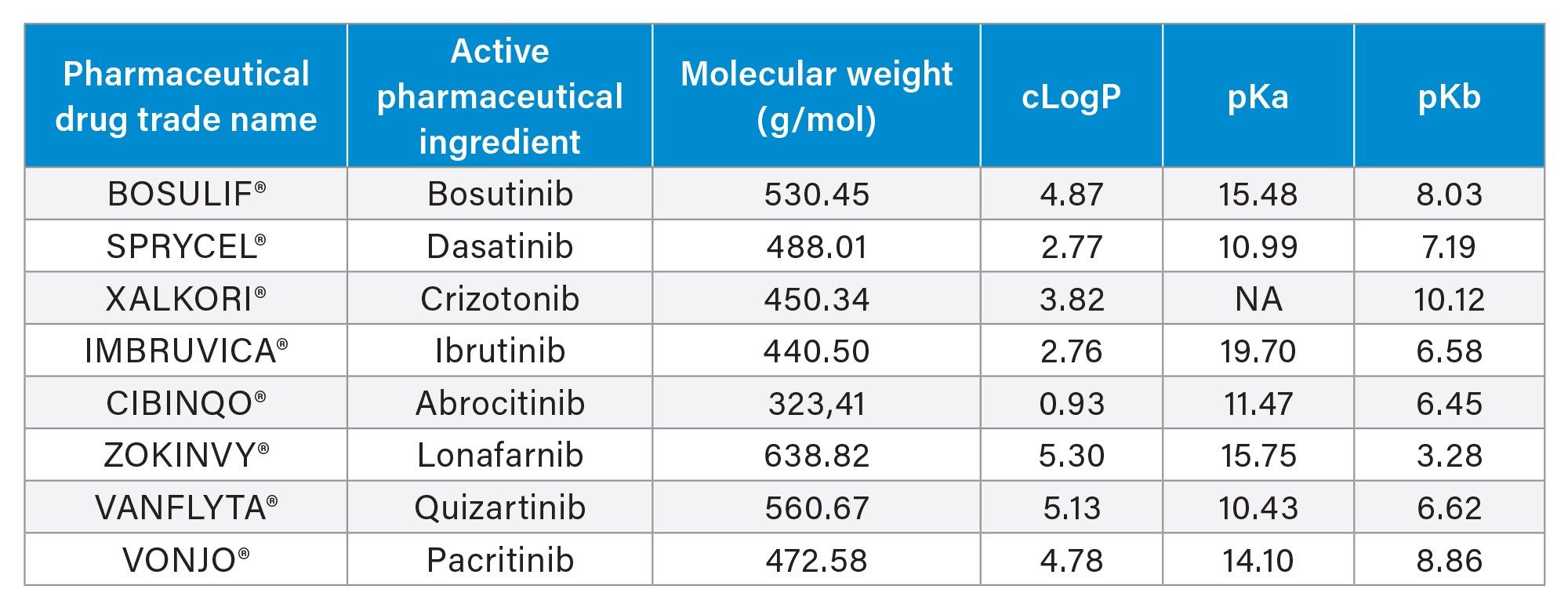
Experimental
UPLC Conditions
|
UPLC: |
I-ClassPlus , FLwith Column Manager (CMA) |
|
MPA: |
0.1% FA in Water |
|
MPB: |
0.1% FA in Acetonitrile |
|
Column: |
ACQUITY UPLC CSH C18 Column, 130 Å, 1.7 μm, 2.1 mm x 30 mm (p/n: 186005295) |
|
Column temperature: |
45 ˚C |
|
Sample temperature: |
15 ˚C |
|
Injection volumn: |
10 μL |
|
Analysis time: |
3.0 min |
|
WNW: |
90:10 Water:ACN+ 0.1% FA |
|
SNW: |
25:25:25:25 Water:IPA:ACN:MeOH |
MS Conditions
|
MS: |
Xevo TQ-XS |
|
|
Capillary (kV): |
0.75 |
|
|
Cone voltage: |
45 V |
|
|
Desolvation temperature: |
500 ˚C |
|
|
Desolvation flow: |
1100 L/Hr |
|
|
Cone gas flow: |
150 L/Hr |
|
|
Source temperature: |
125 ˚C |
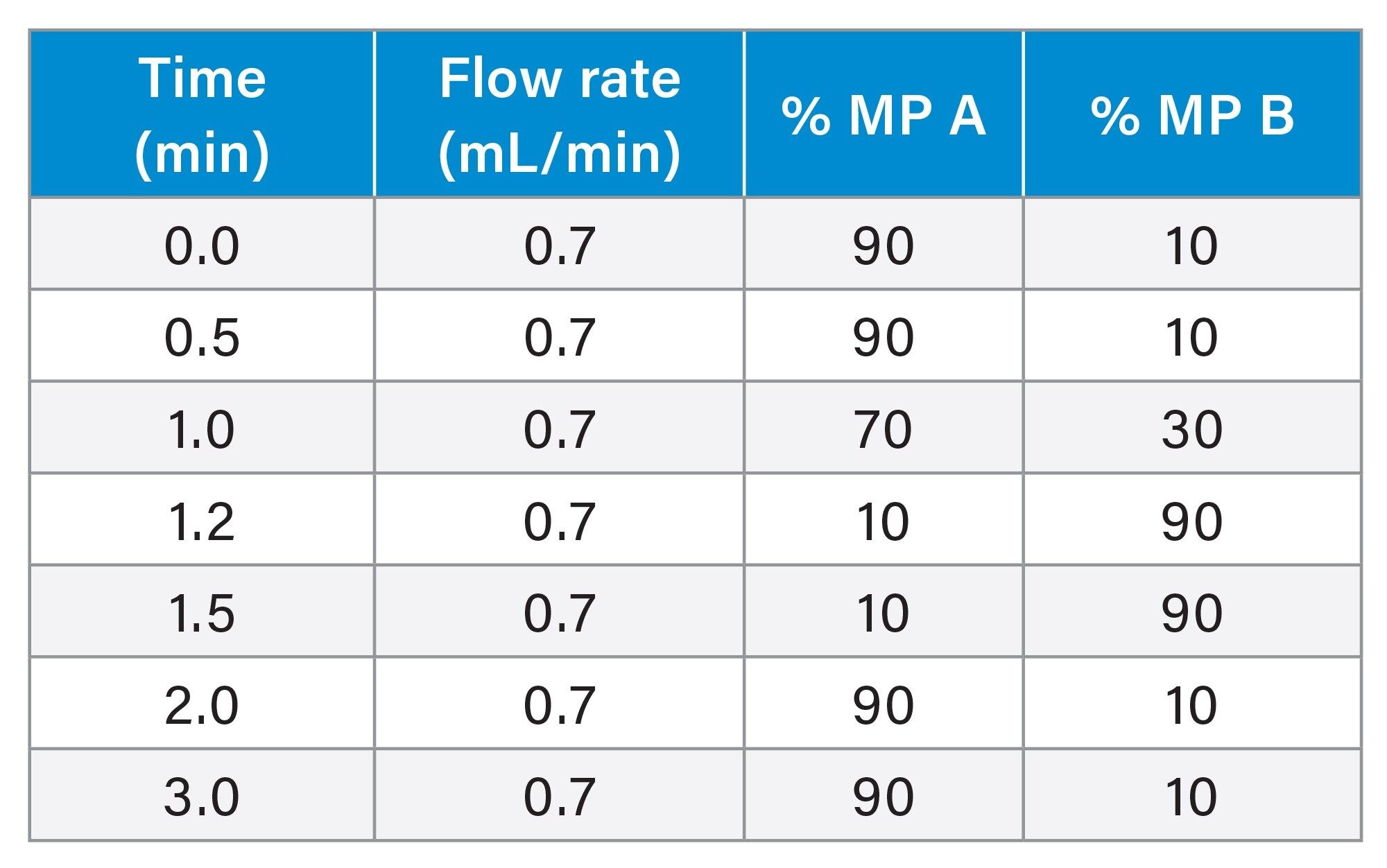
UPLC Gradient Table
Data Management
All data was acquired and analyzed using Waters™ MassLynx™ Software v. 4.1 and quantification performed using TargetLynx™ Software.
Materials
The small molecule pharmaceuticals were purchased from Selleckchem (https://www.selleckchem.com) and from Cayman Chemicals (www.caymanchem.com) LC-MS grade formic acid, and phosphoric acid, and ammonium hydroxide were purchased from Sigma Aldrich (www.sigmaaldrich.com). Methanol and Acetonitrile were purchased from Honeywell (lab.honeywell.com).
Sample Preparation and Extraction
Stock solutions of each individual pharmaceutical (1 mg/mL) were prepared in a 50:50 water: methanol solution. Aliquots of each 1 mg/mL pharmaceutical stock solution were combined to yield working stock solution mixtures of 50, 10, and 1 µg/mL, respectively. Initial SPE recovery and matrix effects evaluations were conducted using 0.5–1.0 µg/mL prepared in rat plasma using the Oasis µElution Sorbent Selection plate and 2 x 4 SPE protocol shown in Figure 1. Final SPE extraction from plasma was performed using the Oasis MCX 96-Well µElution Plate and analysis of the neutral fraction (Elute 1). Calibration curve samples were prepared between 0.91–1,000 ng/mL in plasma (300 µL). Loading of the acid pretreated plasma sample (1:1) was followed by a 200 µL wash of the sorbent with a 2% aqueous formic acid solution followed by a 2 x 25 µL elution of the TKI pharmaceuticals with 100% methanol. SPE extracted eluate was diluted with 50 µL of water prior to LC-MS/MS analysis.
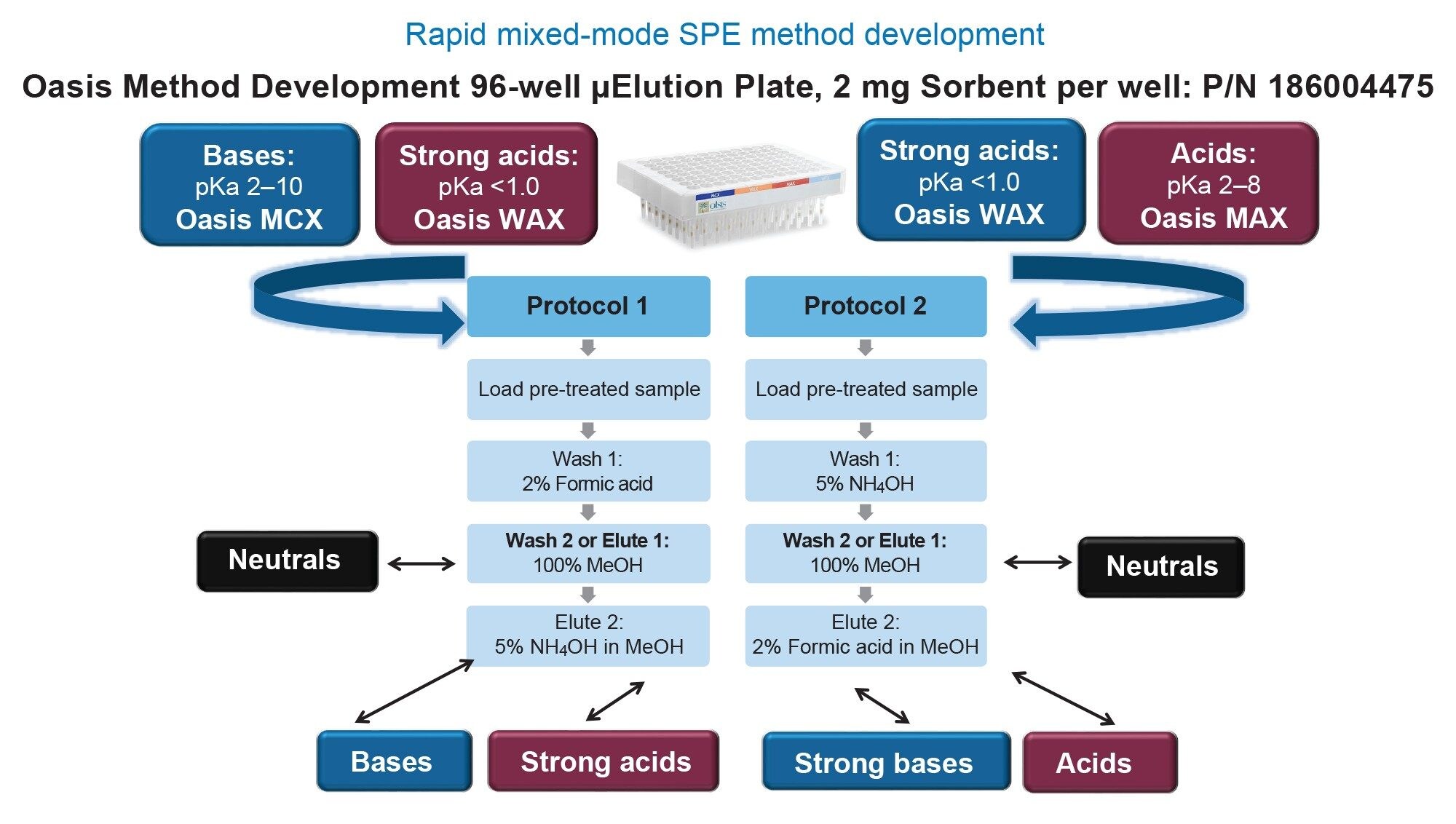
Pharmaceutical analyte recovery was calculated according to the following equation:
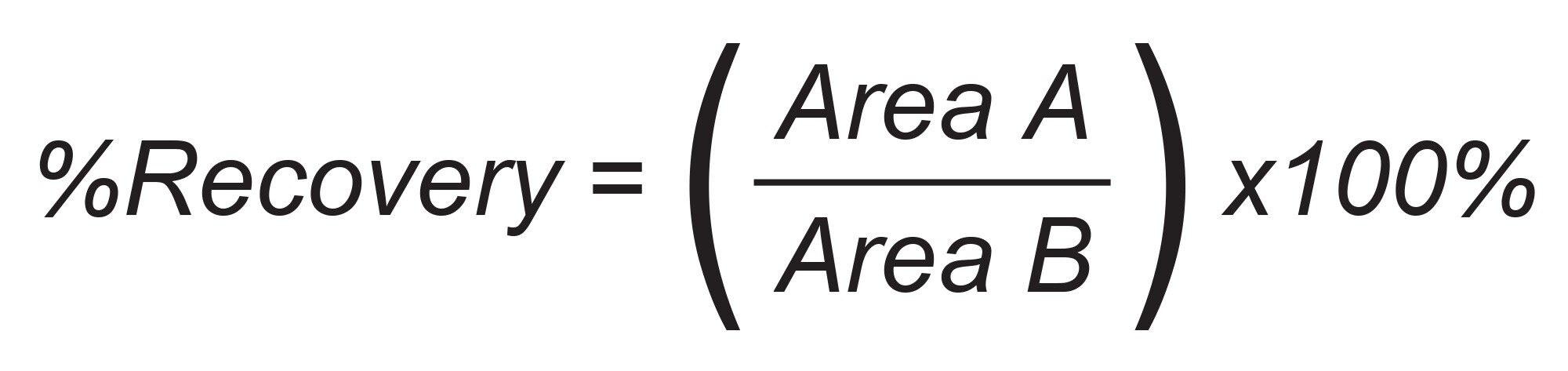
Matrix effects were calculated according to the following equation:

The peak area in the presence of matrix refers to the peak area of an extracted blank matrix sample in which the compounds were added post-SPE extraction. The peak area in the absence of matrix refers to analytes in a neat solvent solution comprised of the final SPE eluate composition injected for analysis. In this case, methanol (neutral elution 1) and 5% NH40H and 2% formic acid in methanol (elution solutions 2) diluted 1:1 with water.
Results and Discussion
LC/MS Analysis
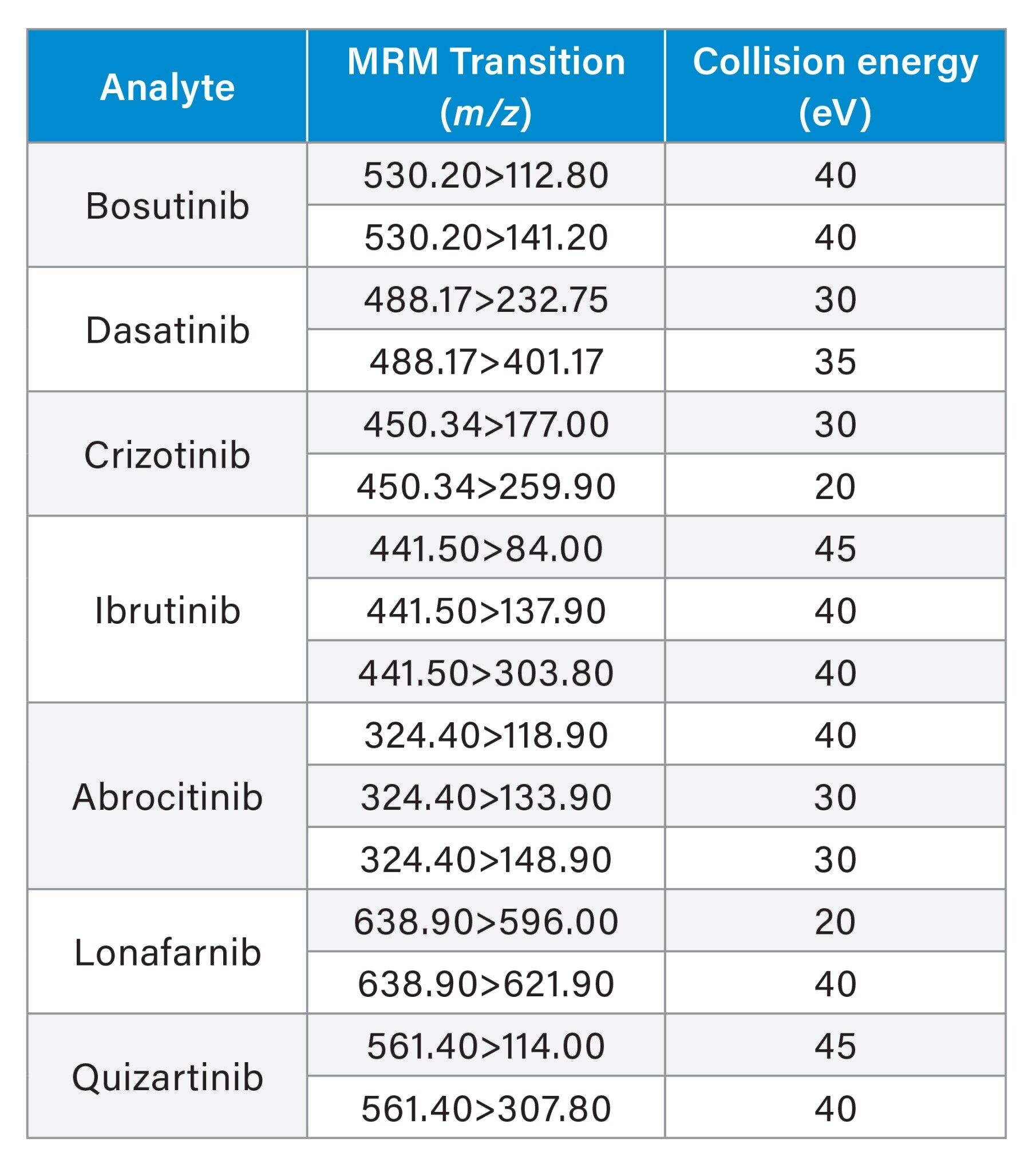
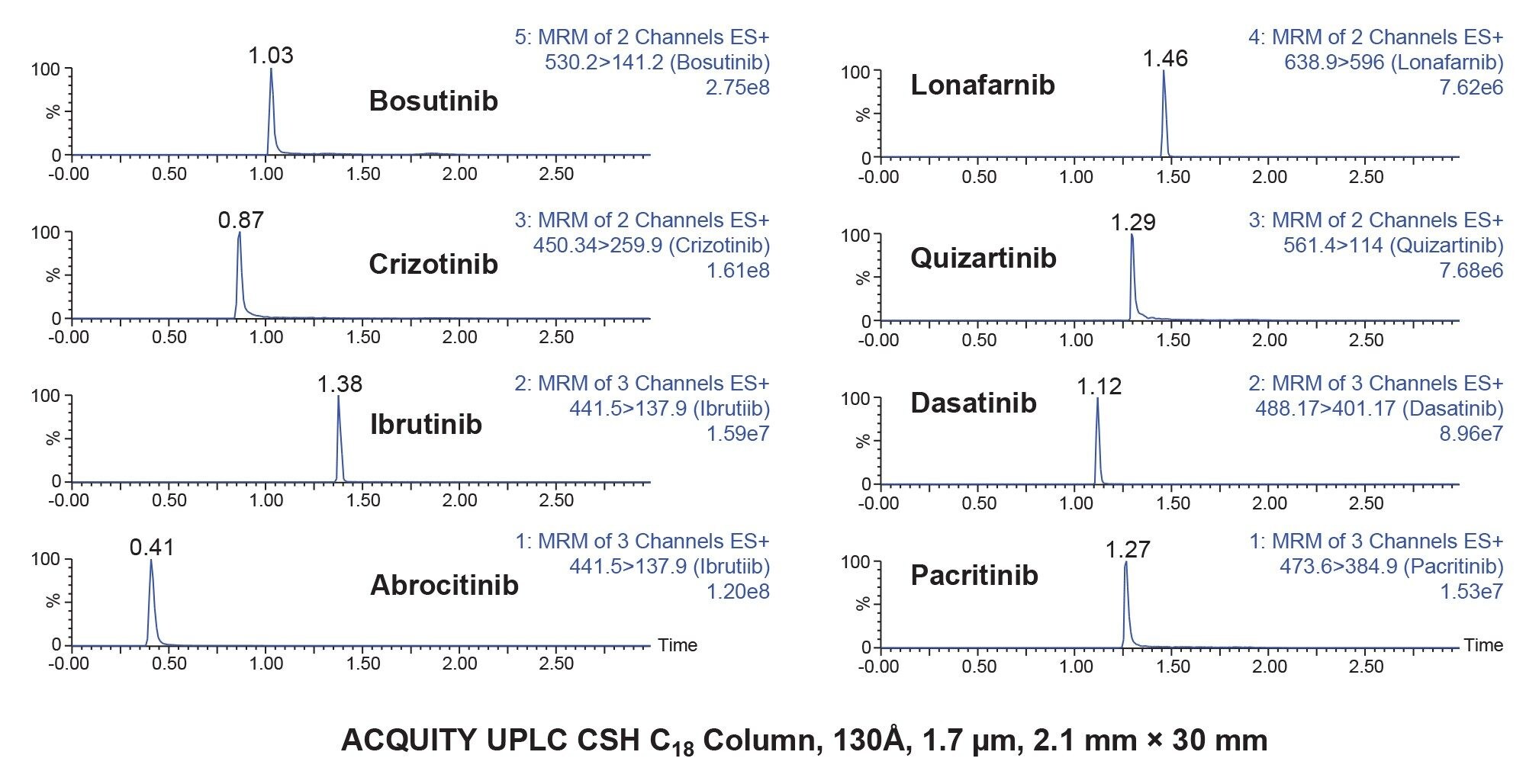
LC-MS/MS analysis was performed using a Waters Xevo TQ-XS Tandem Quadrupole MS using an electro spray ionization (ESI) source and Multi Reaction Monitoring (MRM). The MS MRM transitions for each pharmaceutical are listed in Table 2 Chromatographic separation of these analyte was performed using an ACQUITY I-Class PLUS UPLC System and ACQUITY UPLC CSH C18 Column, 130 Å, 1.7 µm, 2.1 mm x 30 mm (p/n: 186005295). The column temperature was 45 ˚C. Gradient separation using 0.1% formic acid in water, mobile phase A (MP A) and 0.1% formic acid in acetonitrile, mobile phase B (MP B) was employed. Initial LC conditions used a flow rate of 0.7 mL/min and 90% MP A, which was held for 0.5 minutes, followed by an increase to 30% MP B over 0.5 minutes, and then increased to 90% MP B over 0.2 minutes then held at 90% MP B for 0.3 minutes to flush the column and returned to starting gradient conditions at 2.0 minutes. Total analysis time was three minutes. Injection volumes of the extracted samples was 10 µL. An illustration of analyte chromatographic performance is illustrated in Figure 2.
SPE Extraction
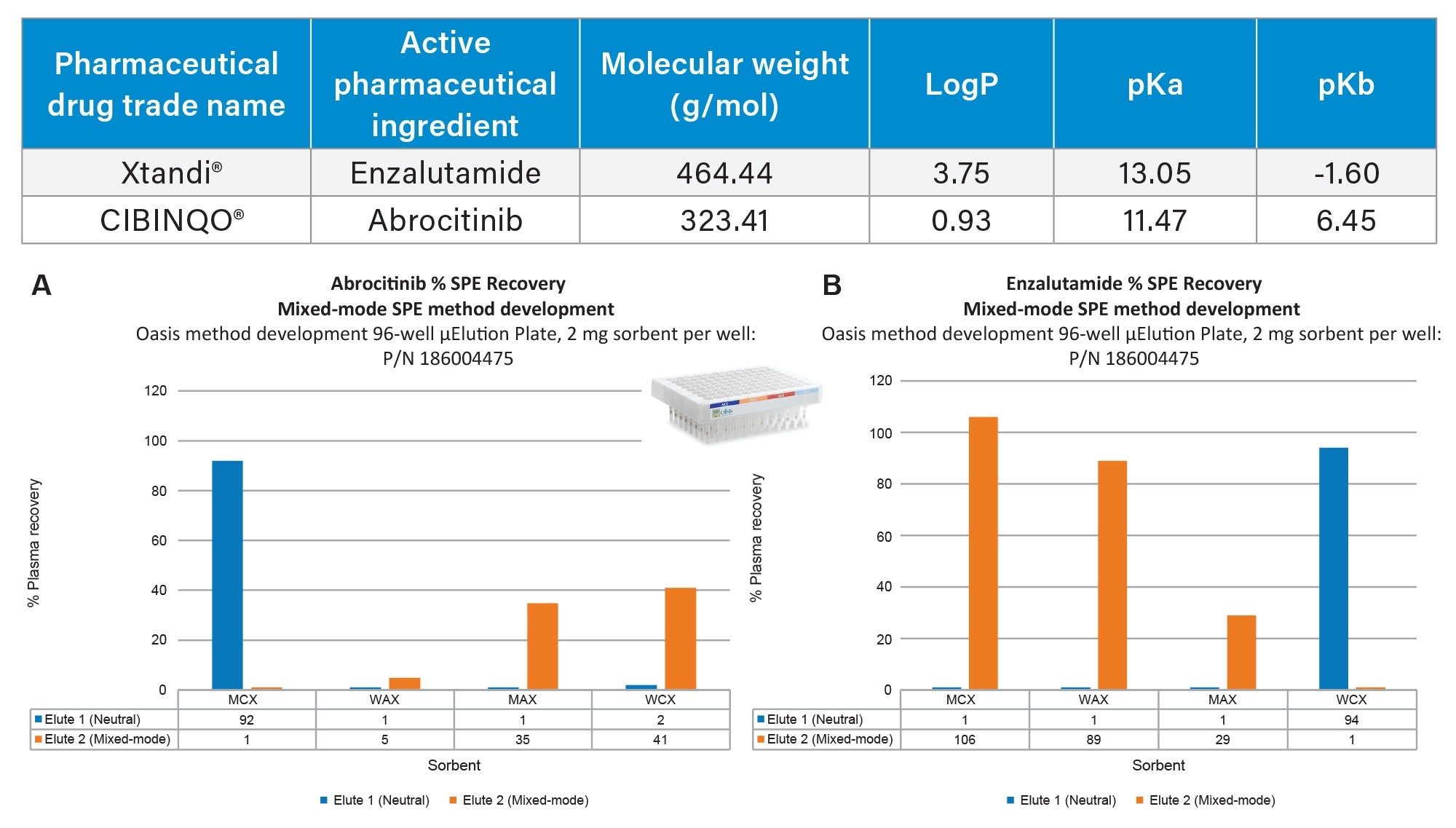
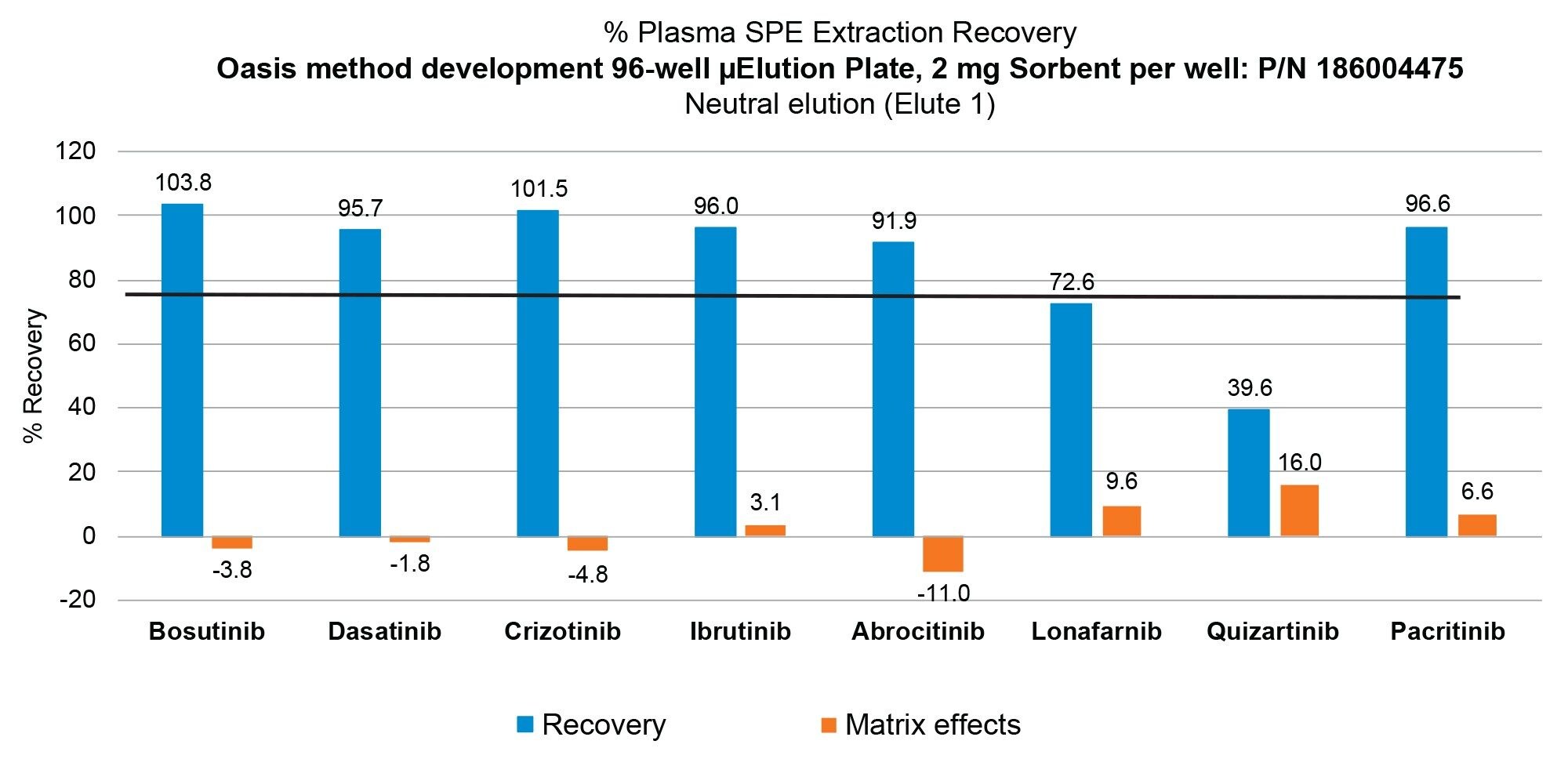
The Oasis mixed-mode sorbents were designed to help scientists achieve the highest level of sample cleanliness and analyte specificity. By combining the power of reversed-phase and ion-exchange retention mechanism, it is possible to design a targeted SPE method by choosing the appropriate Oasis sorbent.3–5 Using the Oasis 2 x 4 Method (Figure 1) in combination with the Oasis Sorbent Selection Plate, containing three rows each of MCX, WAX, WCX, and MAX mixed-mode Oasis SPE sorbents, facilitates fast MM SPE analyte screening in one experiment. Representative MM SPE screening results using this approach is illustrated in Figure 3 for the oncology pharmaceuticals, abrocitinib (A) and enzalutamide (B). In this case, high plasma recovery (100 µL) for both abrocitinib and enzalutamide was found using the MCX sorbent and 2 x 25 µL elution using elution solutions 1 and 2 shown in Figure 1. Abrocitinib eluted in the neutral fraction (elute 1) while enzalutamide recovery was greatest in the mixed-mode fraction (elute 2). The final MCX SPE protocol and plasma recovery and matrix effects for all TKI pharmaceuticals (Table 1) extracted from plasma is shown in Figure 4. With the exception of quizartinib, plasma recovery was greater than 70% from plasma, with no protocol modification required. Using a neat solution standard, quizartinib recovery was >75% (data not shown) pointing to plasma protein binding as the source of low recovery. Future work would focus on modification of the sample pretreatment to effectively disrupt analyte: plasma protein binding to improve quizartinib recovery. In addition to high recovery, high analyte selectivity was achieved with matrix effects for the TKI oncology pharmaceuticals extracted from plasma between -11 and 10%.
Bioanalytical Quantitation
Proof-of-concept bioanalytical quantification of the pharmaceutical analytes extracted from plasma was performed using the Oasis MCX 96-well µElution plate (p/n: 1860001830BA) using 300 µL of plasma pretreated 1:1 with a 4% phosphoric acid solution to aid in protein binding disruption, as well as improve resonance time of the pharmaceutical analytes with the sorbent bed. Loading of the plasma sample was followed by a 200 µL wash of the sorbent with a 2% aqueous formic acid solution followed by a 2 x 25 µL elution of the TKI pharmaceuticals with 100% methanol. SPE extracted eluate was diluted with 50 µL of water prior to LC-MS/MS analysis. Quantification performance from extracted plasma using the Oasis MCX MM sorbent in the µElution 96-well plate format is highlighted in Figure 5. Linear fit of calibration curves was >0.99. Lower limit of quantification (LLOQ) for all pharmaceutics was 0.91 ng/mL, while upper limit of quantification (ULOQ) was 500 ng/mL for bosutinib, crizotinib, and pacritinib. ULOQ for ibrutinib and dasatinib was 1000 ng/mL and 125 ng/mL for abrocitinib . Accuracy of all calibration points was ±15%.
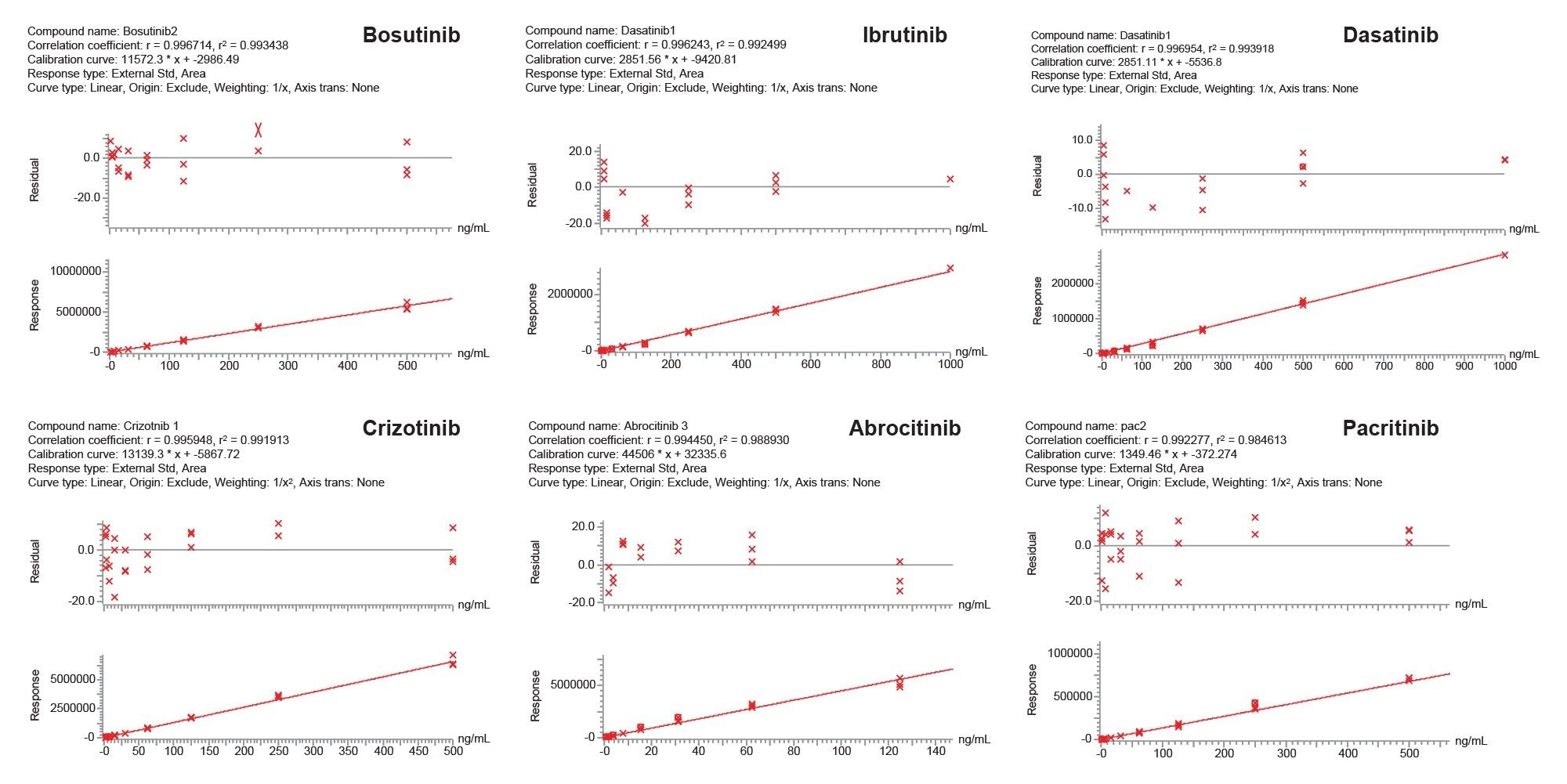
Conclusion
This application highlights the successful SPE extraction and LC-MS/MS quantification of several small molecule TKI oncology pharmaceuticals from plasma using Oasis MCX SPE. Use of the Oasis Method Development Plate and 2x4 SPE protocol strategy greatly simplified MM SPE method development in one experiment, by easily screening four sorbents in one device. Using the Oasis MCX SPE for final bioanalytical extraction from plasma, provided excellent extraction performance, achieving high analyte recovery (70%) and low matrix effects (≤15%), whilst also achieving linear (>0.99) and accurate (15%) quantitation.
References
- Williams, RE and Leatherwood HM Top 200 Small Molecule Drugs by Retail Sales in 2023 https://bpb-us-e2.wpmucdn.com/sites.arizona.edu/dist/9/130/files/2024/05/2023Top200SmallMoleculePosterV5.pdf (accessed 21 June 2024).
- Drugbank Online https://go.drugbank.com/drugs/DB00480 (accessed 21 June 2024).
- Chambers, E, Diehl, D, Mazzeo J, Simple and Fast SPE-UPLC-MS-MS Method for an Allery Tablet Mixture of Acids and Bases https://www.waters.com/webassets/cms/library/docs/wa41942.pdf
- Danaceau, JP, Trudeau ME, A Simple, Broadly Applicable Automated Bioanalytical Sample Preparation Strategy for LC-MS Quantification of Apixaban: Evaluation of Common Bioanalytical Extraction Techniques. Waters Application Note. 720007946. July 2023.
- Rahim F, VanTran, Trudeau ME, Simple, Fast and Selective, Bioanalytical Sample Extraction for the Therapeutic Drug, Lenalidomide From Plasma Using Oasis MCX SPE. Waters Application Note. 720008140. December 2023.
720008447, July 2024