Successful Method Migration of the USP Quetiapine Fumarate Impurities Method to an Alliance™ iS HPLC System
Abstract
In regulated laboratories, method migration is an important aspect to move an established method from one High Performance Liquid Chromatogram (HPLC) System to the same or different HPLC system. Many regulated laboratories need to migrate methods since the laboratory contains varying HPLC instrumentation from the same or different vendors. Additionally, these laboratories may desire to upgrade their older equipment to newer technology to keep up with the increased throughput.1 Successful method migration to a new HPLC system can be challenging due to differences in the instrumentation that may affect the chromatographic results. The Alliance iS HPLC System is designed with many key features and attributes that support method migration that achieves the same if not improved results. In this application note we will look at how the Alliance iS HPLC System has many important elements that will help to achieve a successful method migration. The USP method for Quetiapine Fumarate Impurities will be analyzed on two legacy HPLC systems and then analyzed on the Alliance iS HPLC System to compare and evaluate the method using an unknown sample of the drug substance and the system suitability requirements found in the USP method.2
Benefits
- Straightforward and successful method migration of a USP impurity monograph to the Alliance iS HPLC System
- Reproducible quantitative results of the quetiapine fumarate drug substance
- Increased injection precision achieved on the Alliance iS HPLC System
Introduction
During method migration it is important to achieve consistent, reproducible results across varying HPLC systems. Differences in the HPLC systems’ design can generate variations in the chromatographic results. In this study, the gradient USP method for Quetiapine Fumarate Impurities will be analyzed on two legacy HPLC systems along with the new technology of the Alliance iS HPLC System. On each HPLC system, the system suitability requirements for the area and retention time %RSD, tailing factor and the resolution of two critical peaks will be evaluated. Along with the system suitability requirements, a sample of the quetiapine fumarate drug substance will be analyzed for impurities to demonstrate the consistent quantitative results are generated across all three HPLC systems. The results of the study will demonstrate how the Alliance iS HPLC System allows for method migration to modern technology.
Experimental
Sample Description
The USP method requires a quetiapine fumarate reference standard and a system suitability reference standard. The quetiapine standard solution was prepared using the USP quetiapine fumarate reference standard (catalog: 1592704) and was prepared at a concentration of 0.001 mg/mL in diluent (86:14 Solution A: Solution B). The system suitability solution was prepared from the USP quetiapine system suitability RS (USP p/n: 1592715) and consists of a mixture of quetiapine, quetiapine desethoxy (1–5%), related compound G and related compound B standard. The system suitability solution was prepared at 1 mg/mL in diluent (86:14 Solution A: Solution B).
The quetiapine drug substance was obtained from Hangzhou Think Chemical Co., Ltd. and past the date of expiration. The sample was prepared at 1.0 mg/mL in Solution A.
LC Conditions
|
LC system: |
Alliance e2695, HPLC System 2, Alliance iS HPLC System |
|
Detection: |
UV/Vis detection for all systems |
|
Wavelength: |
250 nm |
|
Column: |
XBridge BEH C8, 3.5 µm, 4.6 x 150 mm (p/n: 186003055) |
|
Column temperature: |
45 °C |
|
Sample temperatrure: |
10 °C |
|
Injection volume: |
20 µL |
|
Flow rate: |
1.50 mL/min |
|
Mobile phase A: |
Solution A: Acetonitrile and Buffer (75:25) |
|
Mobile phase B: |
Solution B: Acetonitrile |
|
Buffer: |
3.1 g/L of ammonium acetate in water. Two mL of 25% ammonium hydroxide was added to each 1 Litre of solution. The final pH is not less than (NLT) 9.2 |
|
Seal wash: |
90:10 Water: acetonitrile |
|
Purge solvent: |
50:50 Water: acetonitrile |
|
Wash solvent: |
50:50 Water: acetonitrile |
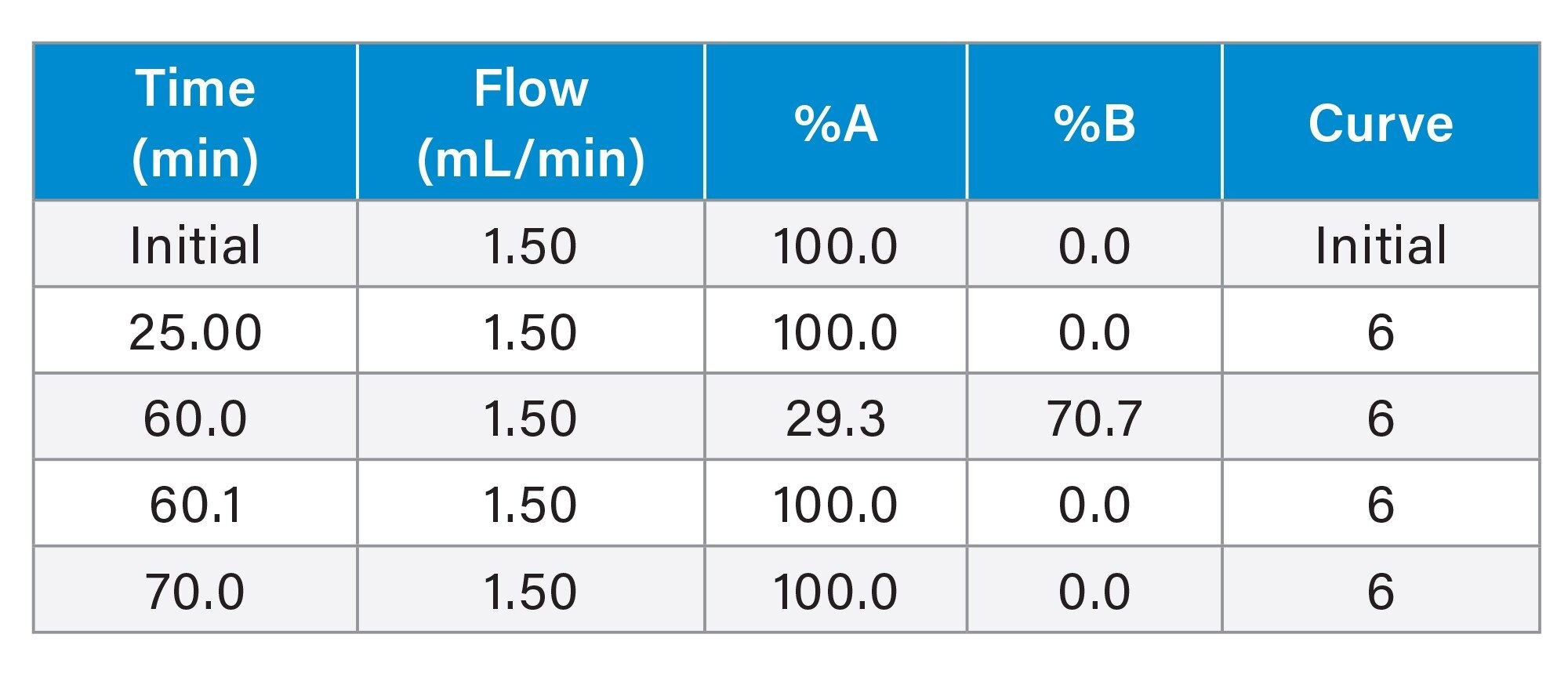
Gradient
Data Management
|
Data management: |
Empower 3 Chromatography Data Software |
Results and Discussion
The introduction of modern HPLC systems into a regulated laboratory requires that the data and results from the old HPLC system are replicated on the new, modern HPLC system. Using the Alliance iS HPLC System provides many similar instrument characteristics, such as a quaternary solvent manager, a flow-thru-needle (FTN) sample manager, and a TUV detector, that are commonly found in legacy HPLC systems, with improvements in usability and designs. These characteristics provide the same quantitative results with the ability to achieve similar and/or improved system suitability results as these legacy systems. In this application we will demonstrate how the Alliance iS HPLC System compares to two legacy HPLC systems for the analysis of the USP monograph for Quetiapine Fumarate Impurities, a gradient method. To assess the success of the method migration, the system suitability requirements will be analyzed. In addition, the analysis and quantitation of the organic impurities of a drug substance will be performed. All the results and analyses will follow the USP method criteria and be based on six replicate injections.
System Suitability for Quetiapine Standard: Injection Precision and Repeatability
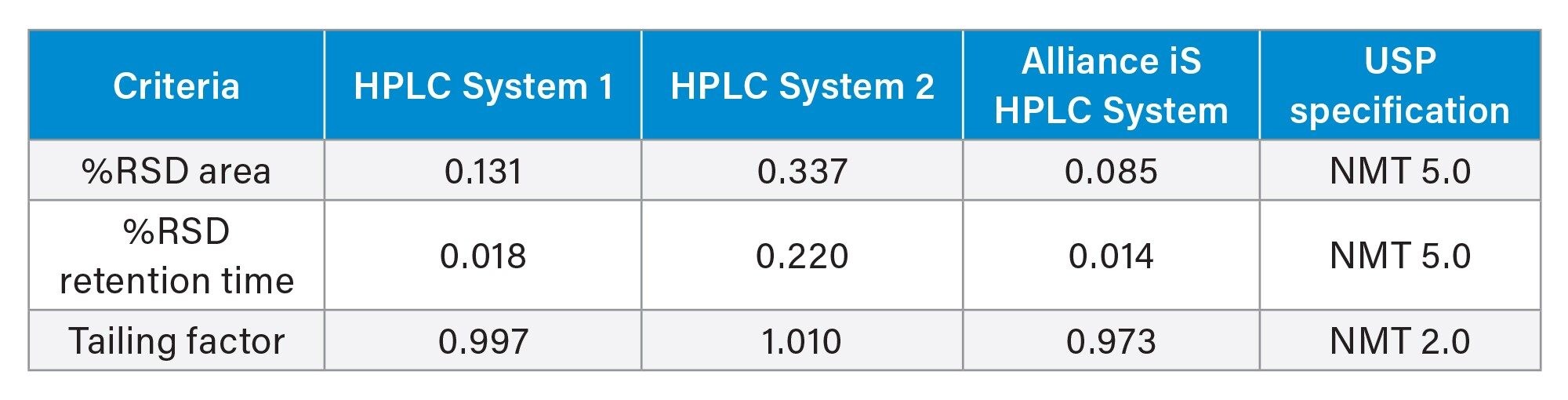
System suitability requirements are an important part of every USP method, to ensure that the LC system is suitable to complete the USP method. The USP Quetiapine Fumarate Impurities method system suitability requirements include two criteria for the quetiapine fumarate standard solution: relative standard deviation (%RSD) for area and retention time of not more than (NMT) 5.0%. All three HPLC systems met the %RSD requirements for area and retention time without difficulty (Table 1).
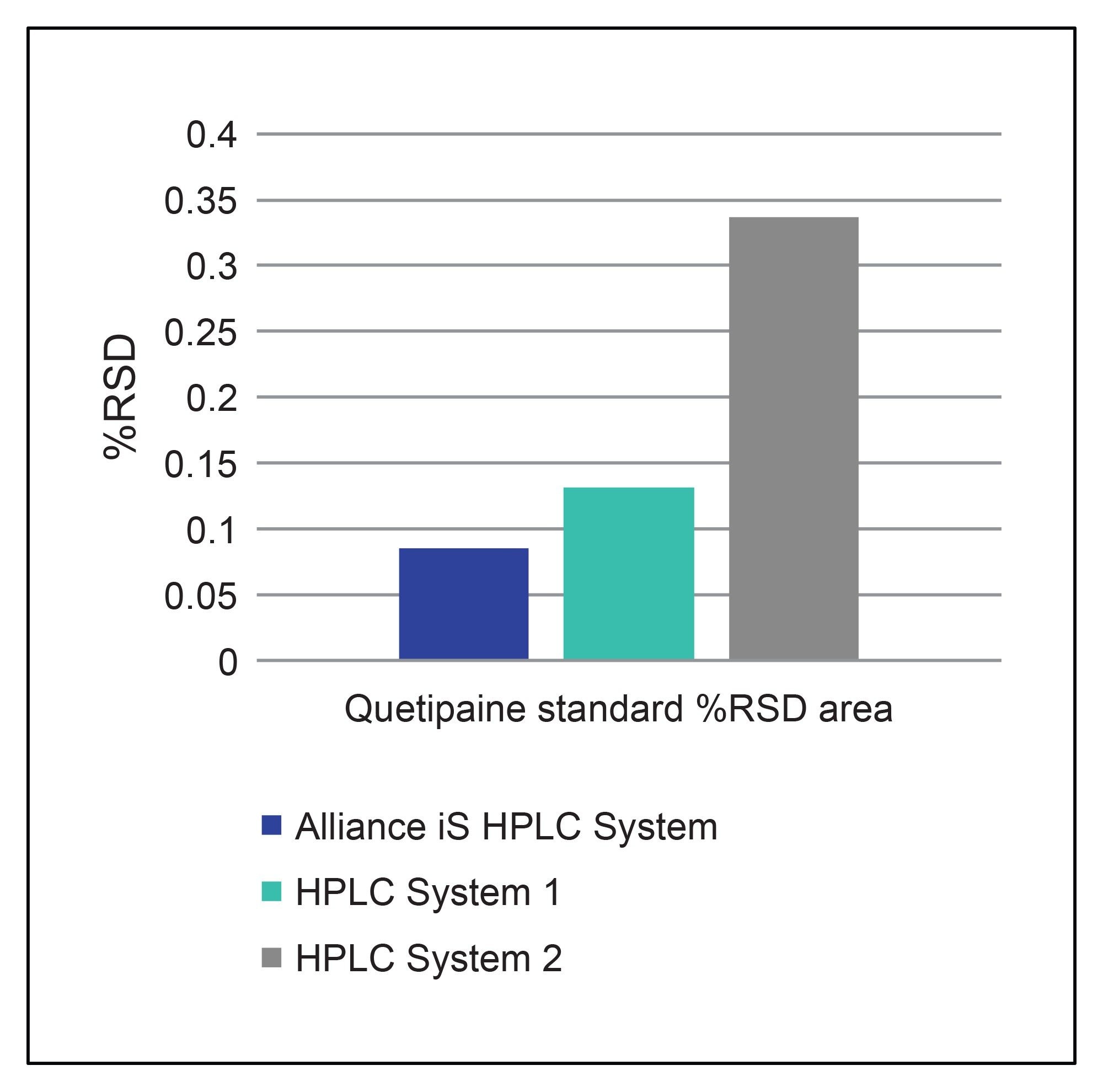
One important system performance measure for an HPLC system is injection precision. Since the system suitability requirements demonstrate how a system is performing, the %RSD for area demonstrates the precision of the injection mechanism under the prescribed method conditions. All three HPLC systems showed good %RSD values which met the requirement of 5%, but the Alliance iS HPLC System had the best results out of the three systems. The Alliance iS HPLC System includes a redesigned injection mechanism which now includes a high-pressure sample metering device. This enabled the system to achieve an area RSD of only 0.085%. The system suitability requirements for %RSD for area obtained on the Alliance iS HPLC System demonstrates an improvement in the injection precision values over the other two HPLC systems (Figure 1) with the newly designed sample metering device.
Additionally, the system suitability requirements for the standard solution include the %RSD for retention time (RT) and tailing factor. The repeatability of the three HPLC systems was demonstrated with the %RSD for RT and all three systems showed good repeatability (Table 1). All three HPLC systems performed well for tailing factor and were well within the system requirements of no less than (NLT) 2.0 (Table 1).
USP System Suitability Calculations Revised for Resolution
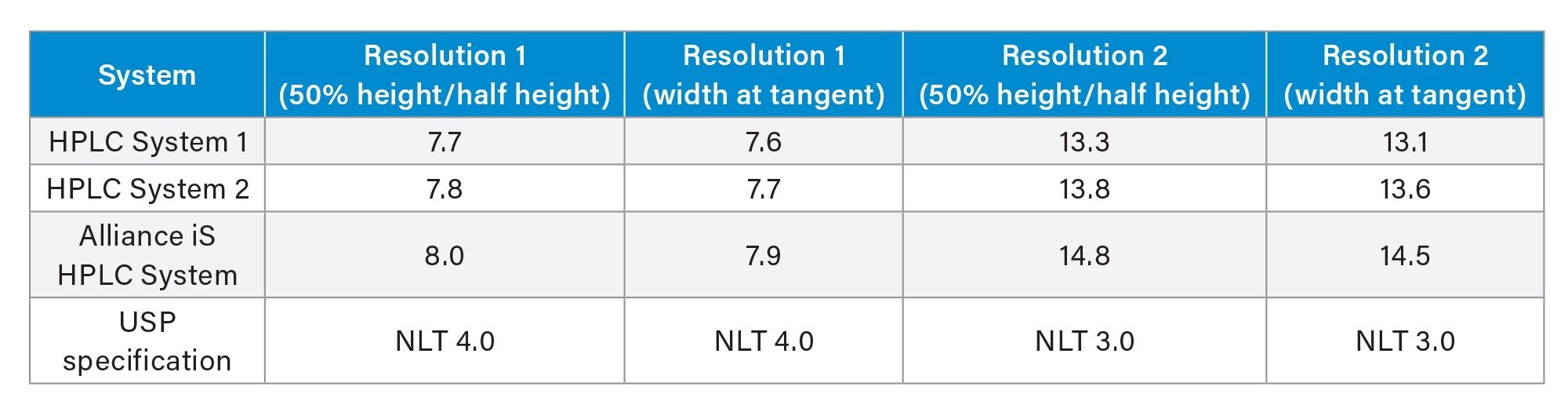
Recent changes to USP General Chapter <621> impact the methodology used for calculation of the resolution.3 The revised version of <621> requires that resolution of two peaks is calculated at peak half-height, whereas previously the calculation was based on the tangent width of the peak.4 The resolution for the two critical pairs in the Quetiapine Fumarate Impurities method was calculated using both the revised calculation and the previous calculation (Table 2). Based on the data collected, the change in calculation of resolution did not significantly impact the system suitability results since all calculated values were very similar for all HPLC systems. Resolution 1 is the critical pair of quetiapine desethoxy (peak 3) and quetiapine (API, peak 4). Resolution 2 is the critical pair of Related Compound G (peak 1) and Related Compound B (peak 2). When comparing Resolution 1 and Resolution 2 on all three HPLC systems, the Alliance iS HPLC System showed the greatest resolution (Figure 2).
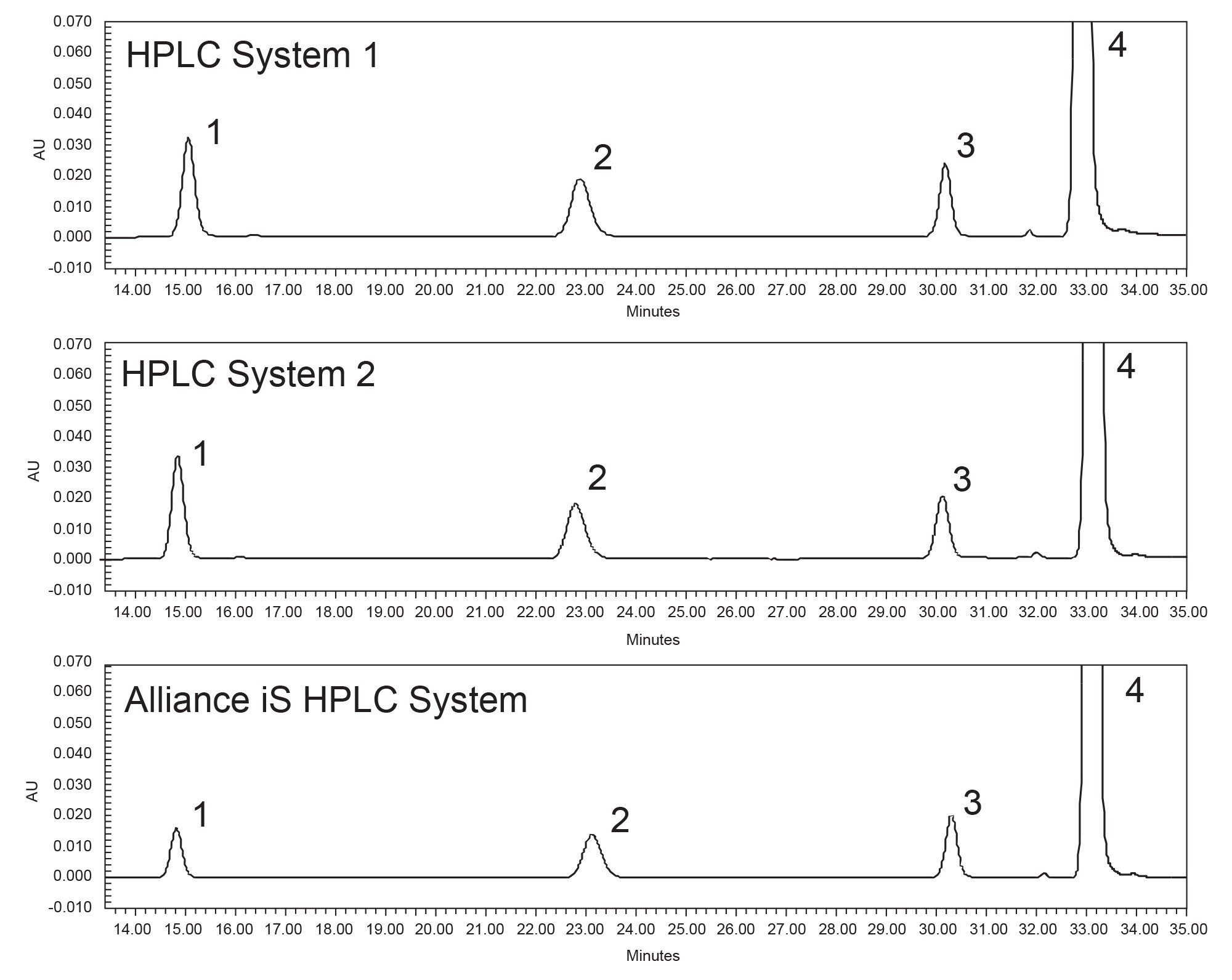
Quantitative Results of the Drug Substance
One important aspect of migrating a method from one system to another is confirming that the same quantitative results are obtained on all the systems. The USP monograph for Quetiapine Fumarate Impurities determines the relative amount of impurities in the drug substance. Therefore, it is imperative that the quantitative data is consistent across all systems on which the method is being analyzed. The quetiapine fumarate drug substance was tested on all three HPLC systems. Individual sample preparations were performed for each of the analyses on each of the three HPLC systems (Figure 3). The drug substance analysis on Alliance e2695, HPLC System 2, and the Alliance iS HPLC System resulted in similar quantitative data, all within 0.01% (Table 3).
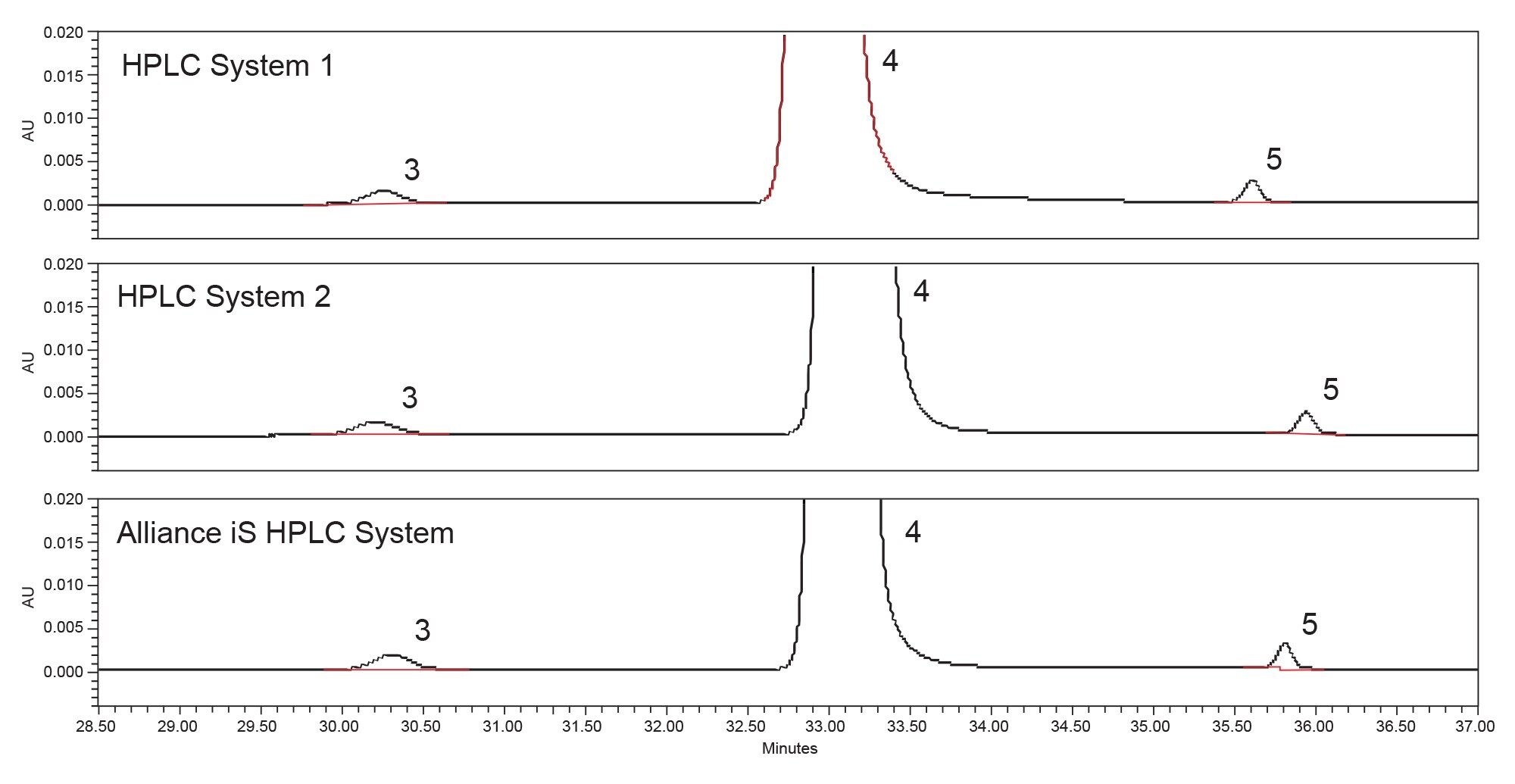
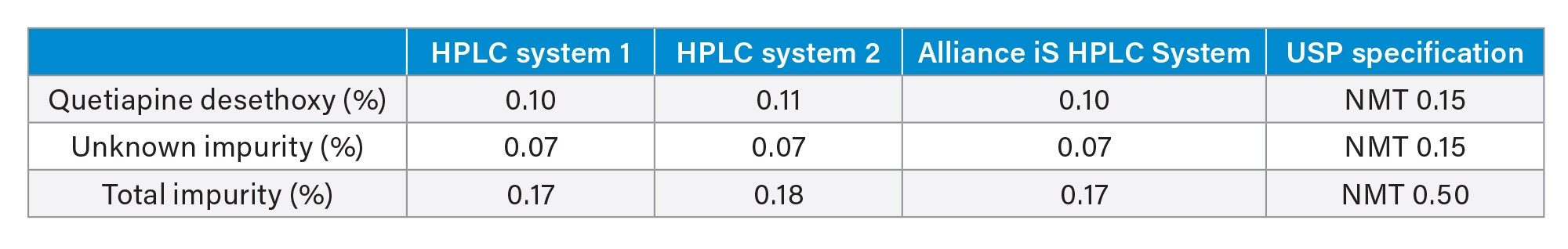
Conclusion
Method migration is essential in regulated laboratories in order to achieve consistent, reproducible results of well-established HPLC methods. In this application note, the USP Quetiapine Fumarate Impurities method was migrated across three HPLC systems, two legacy HPLC systems, and the new Alliance iS HPLC System. All three HPLC systems met the system suitability requirements without any difficulty and the Alliance iS HPLC System showed improved injection precision and improved resolution of two critical pairs. One key requirement of successful method migration is obtaining the same quantitative results on all systems. In this study, all three HPLC systems showed comparable quantitative results for the quetiapine fumarate drug substance. The data demonstrates that the Alliance iS HPLC System can be used for method migration of established HPLC methods for a regulated laboratory.
References
- Stephanie Harden and Phil Nethercote. Error Mitigation in Pharmaceutical Quality Assurance and Control. Innovations in Pharmaceutical Technology. 13 Dec 2022.
- GUID-DBEED03E-7C75-4167-BD21-4E30BA2EFF2B_2_en-US (Quet Imp. Method).
- GUID-6C3DF8B8-D12E-4253-A0E7-6855670CDB7B_6_en-US (USP 621 current).
- GUID-6C3DF8B8-D12E-4253-A0E7-6855670CDB7B_1_en-US (USP 621 old version).
720007944, Revised May 2024