This technology brief demonstrates the sensitive and robust HRMS quantification of trastuzumab from plasma.
The Xevo TQ-XS Tandem Quadrupole is compared with Xevo G2-XS QTof for the bioanalytical quantification of trastuzumab prepared from plasma. For HRMS quantification, best sensitivity and performance was achieved using Tof-MRM mode. In addition, this performance was highly comparable to the Xevo TQ-XS Tandem Quadrupole MS results, achieving LLOQs within 2-fold and 4-orders of linearity. This highly reproducible data demonstrates that the Xevo G2-XS QTof System can be used to provide sensitive, accurate, and robust quantitative results.
With the increased focus on developing proteins as new drug candidates, particularly monoclonal antibodies (mAbs), there is great demand for sensitive and robust quantitative bioanalytical methods. With its fast method development times, broad linear dynamic range, sensitivity, and specificity, LC-MS analysis using a tandem quadrupole MS is quickly becoming an attractive alternative to immunoassays. While tandem quadrupole MS systems have traditionally been the ‘go-to’ instrument for bioanalytical quantification, HRMS instruments can now achieve sensitivities and dynamic ranges that are comparable to that of tandem quadrupole instruments.
In addition, HRMS offers better selectivity, providing improved signal:noise (S:N) compared to a unit resolution tandem quadrupole MS and the ability to collect both qualitative and quantitative results in a single analysis. In this work, we demonstrate the sensitive and robust HRMS quantification of trastuzumab from plasma which achieves performance comparable to tandem quadrupole MS.
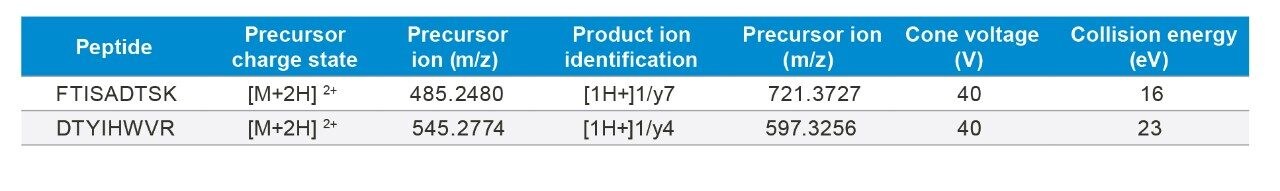
The quantitative performance of the high resolution, Xevo G2-XS QTof Quadrupole Time-of-Flight (Tof) Mass Spectrometer was compared to the nominal mass Xevo TQ-XS Tandem Quadrupole Mass Spectrometer for the quantification of trastuzumab in plasma. The unique tryptic peptides of trastuzumab, FTISADTSK and DTYIHWVR, were used for this assessment. Their peptide sequences, precursors, product ions, and corresponding charges states are listed in Table 1. For HRMS analysis, several experiments were performed on the Xevo G2-XS QTof, including: Tof-MRM with target enhancement of the product ion (precursor > fragment), Tof-MRM with target enhancement of the precursor ion (precursor > precursor), and a Tof-MS full scan acquisition of precursor ions (full scan > precursor).
A multiple reaction monitoring (MRM) experiment was performed for tandem quadrupole MS analysis on the Xevo TQ-XS System. Chromatographic separation was achieved using an ACQUITY UPLC H-Class System and an ACQUITY UPLC Peptide BEH C18 Column (p/n: 186003687), using an eight minute gradient (5–50% B) with 0.1% formic acid in water and acetonitrile (flow rate 0.3 mL/min). Trastuzumab was immunopurified from plasma (50 μL) using a 96-well Protein A agarose-based plate. The postaffinity purified plasma was then digested and peptide-level purification was completed using the ProteinWorks eXpress Digest and μElution SPE Clean-up Kits (p/n: 176003689 and p/n: 186008304). An 8 μL aliquot of the resulting 90 μL SPE eluate was injected for each LC-MS analysis.
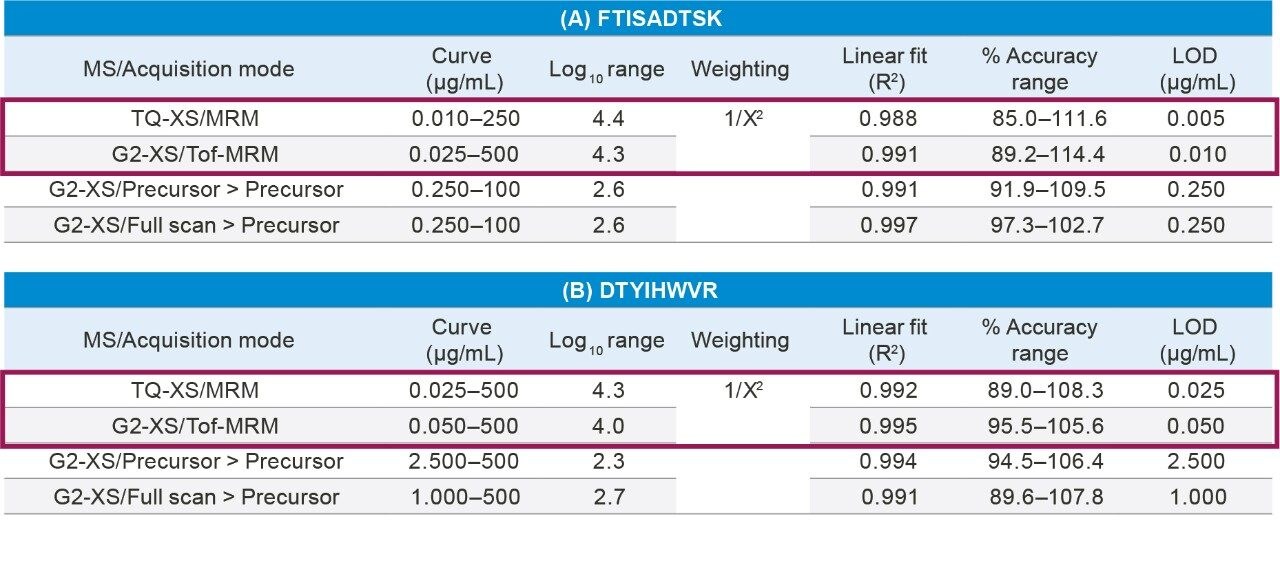
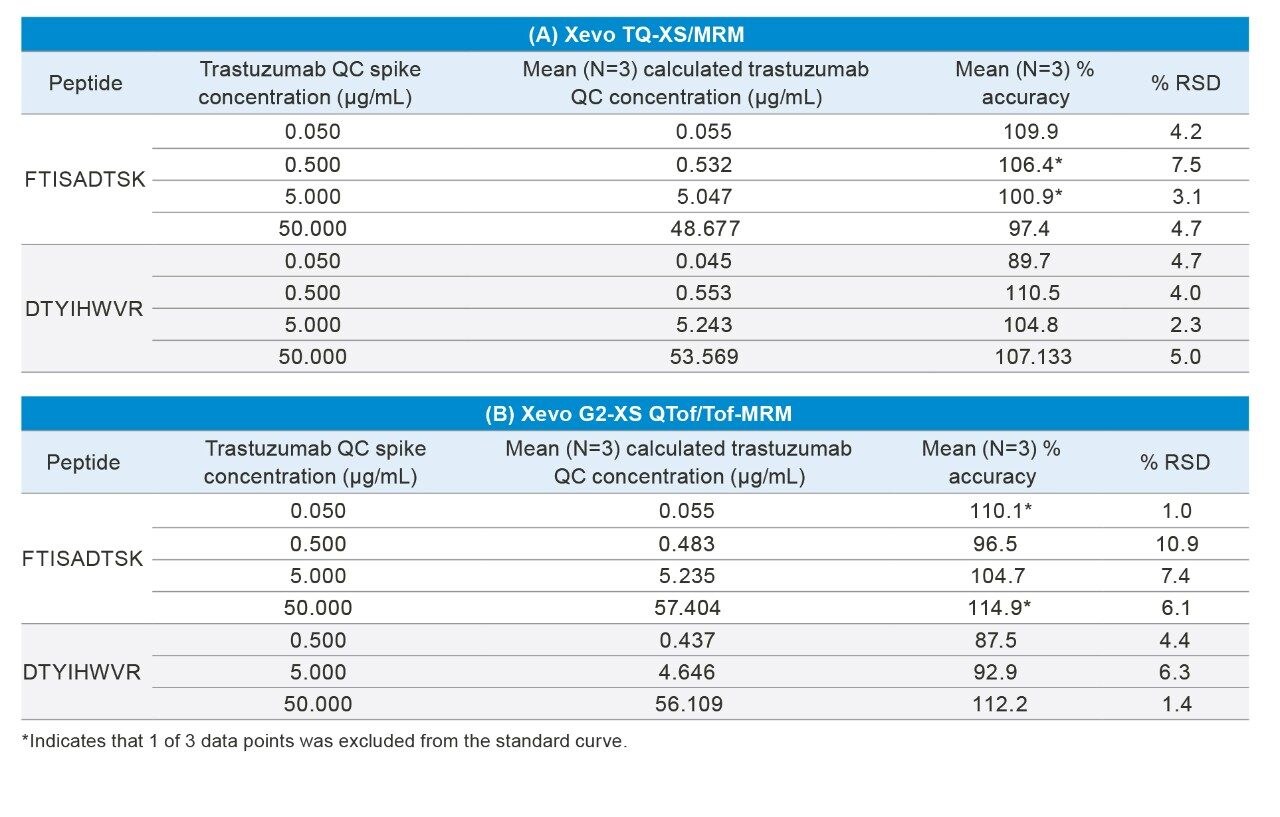
A summary of standard curve performance for the FTISADTSK and DTYIHWVR peptides using both the Xevo TQ-XS and Xevo G2-XS QTof with the various acquisition modes is highlighted in Table 2. Best overall quantification performance was achieved using the tandem quadrupole Xevo TQ-XS MS, with lower limits of quantification (LLOQs) between 10–25 ng/mL and linear dynamic range ≥4.3 orders of magnitude. While all three HRMS modes on the Xevo G2-XS QTof MS showed excellent linearity (R2 values ≥0.99), best sensitivity and performance, which was comparable to the Xevo TQ-XS, was achieved using Tof-MRM with linear dynamic range ≥4.0 orders of magnitude and LLOQs between 25–50 ng/mL. QC performance, highlighted in Table 3, was excellent for both tandem quadrupole and HRMS MRM analysis with mean accuracies and % CV’s ±15%. Chromatographic performance for the FTISADTSK tryptic peptide of trastuzumab is highlighted in Figure 1.
In this work we compared the Xevo TQ-XS Tandem Quadrupole Mass Spectrometer to the Xevo G2-XS QTof Quadrupole Time-of-Flight Mass Spectrometer for the bioanalytical quantification of trastuzumab prepared from plasma. For HRMS quantification, best sensitivity and performance was achieved using Tof-MRM mode. In addition, this performance was highly comparable to the Xevo TQ-XS Tandem Quadrupole MS results, achieving LLOQs within 2-fold and 4-orders of linearity. This highly reproducible data demonstrates that the Xevo G2-XS QTof System can be used to provide sensitive, accurate, and robust quantitative results.
720006207, March 2018