
For research use only. Not for use in diagnostic procedures.
In this study, we will demonstrate the transfer of an RP method across ACQUITY Arc Bio System and Agilent 1260 Infinity Bio-inert System. We will show retention time and peak area percent repeatability across these two LC systems. A column, specifically designed for separation of intact monoclonal antibody (mAb) and subunits, Waters BioResolve RP mAb Polyphenyl Column, will be used for the study. The chromatographic system characteristics, including system dispersion and dwell volume, will be evaluated on their impact on resulting chromatography. In the end, we will explain the features of the ACQUITY Arc Bio System, Multi-flow path Technology and Gradient SmartStart and how they enable the gradient delay of other systems to be emulated without the need for adjustments to the gradient table.
Biopharmaceutical companies often need to transfer analytical liquid chromatography (LC) methods within their own organization and/or to external contract organizations. Among these LC methods, reversed phase (RP), size-exclusion chromatography (SEC), and ion exchange (IEX) are commonly used to characterize biomolecules. Biocompatible or bio-inert LC systems are frequently chosen for SEC and IEX analysis due to conditions requiring aqueous mobile phase with high salt concentrations. The materials used to build biocompatible or bio-inert LC systems typically include MP35N, a nickel-cobalt alloy, titanium, or polyether ether ketone (PEEK), just name a few. Those materials can reduce potential corrosion from high salt concentration and avoid oxidation of the protein by the presence of iron ions. In many cases, the same LC systems would be employed for RP analysis as well. RP is used to support various stages of biopharmaceutical development because of its resolving power and amenability to mass spectrometric (MS) detection. Multiple manufacturers make biocompatible and bio-inert LC systems. Therefore it is important that method transferability from system to system is demonstrated to minimize lost productivity, such as re-developing methods, failing batches, etc.
In this study, we will demonstrate the transfer of an RP method across ACQUITY Arc Bio System and Agilent 1260 Infinity Bio-inert System. We will show retention time and peak area percent repeatability across these two LC systems. A column, specifically designed for separation of intact monoclonal antibody (mAb) and subunits, Waters BioResolve RP mAb Polyphenyl Column, will be used for the study. The chromatographic system characteristics, including system dispersion and dwell volume, will be evaluated on their impact on resulting chromatography. In the end, we will explain the features of the ACQUITY Arc Bio System, Multi-flow path Technology and Gradient SmartStart and how they enable the gradient delay of other systems to be emulated without the need for adjustments to the gradient table.
A mAb Subunits Standard (Waters, p/n 186008927) containing 25 µg of reduced, IdeS-digested NIST mAb (Reference Material 8671) was dissolved in 100 µL water. The final concentration was 0.25 mg/mL.
LC system: ACQUITY Arc Bio System and Agilent 1260 Infinity Bio-inert System
|
Systems |
ACQUITY Arc Bio |
Agilent 1260 Infinity Bio-inert |
|---|---|---|
|
Pump |
QSM-R Bio, Path 1 and Path 2 |
1260 Bio Quat (G5611A) |
|
Injector |
FTN-R Bio |
1260 HiP Bio ALS (G5667A) |
|
Column compartment |
30 cm CH with standard bio passive preheater |
1290 TCC (G1316C) with 9 μL bio heat exchanger |
|
Detector |
2998 (PDA) with inert 10 mm flow cells |
1260 DAD VL+ (G1315C) with inert 10 mm flow cell |
|
Column: |
BioResolve RP mAb Polyphenyl, 450Å, 2.7 μm, 4.6 × 50 mm (p/n 176004167: includes Column, Intact mAb and mAb Subunit Standards) |
|
Column temp.: |
65 °C |
|
Sample temp.: |
4 °C |
|
Injection volume: |
19.2 μL |
|
Flow rate: |
0.96 mL/min |
|
Mobile phase A: |
0.1% Trifluoroacetic acid (TFA) in water |
|
Mobile phase B: |
0.1% TFA in acetonitrile |
|
Wavelength: |
280 nm |
|
Sampling rate: |
10 Hz |
|
Filter time constant: |
Normal (0.2 sec) for ACQUITY Arc Bio System; response time 0.5 sec for Agilent Infinity 1260 Bio-inert System |
|
Chromatography software: |
Empower 3 FR3 and OpenLab Chemstation |
|
Time (min) |
Flow rate (mL/min) |
%A |
%B |
|---|---|---|---|
|
Initial |
0.96 |
85 |
15 |
|
20.0 |
0.96 |
45 |
55 |
|
20.3 |
0.96 |
20 |
80 |
|
21.3 |
0.96 |
20 |
80 |
|
21.6 |
0.96 |
85 |
15 |
|
25.0 |
0.96 |
85 |
15 |
Data acquisition was performed using the system’s native software and processed in Empower. The Waters Data Converter was used to transfer the OpenLab Chemstation CDS data into Empower 3 FR3.
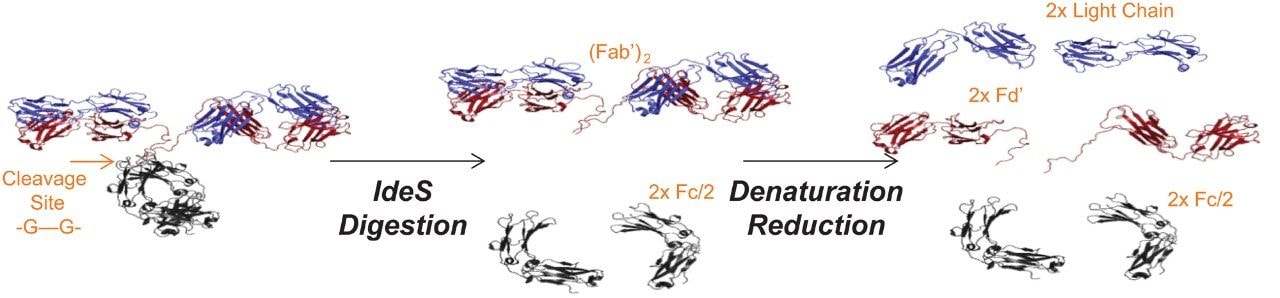
Reversed phase analyses provide separation based on the hydrophobicity of molecules. Depending on the polarity of the analyte, ion-pairing reagents can also be used to assist in retention. For proteins, this technique can be used to separate intact mAb and its subunits, which have very different hydrophobicities. However, RP analysis of proteins can be challenging due to the size and complexity of the intact proteins and their subunits. The Waters BioResolve RP mAb Polyphenyl Column is designed with polyphenyl ligand to offer favorable desorption and retentitivity. Superficially-porous and large pore size particles in stationary phase improve kinetic performance.1 Thus, the BioResolve RP mAb Polyphenyl Column is chosen for the mAb subunits analysis using IdeS treated NIST mAb as a representative sample. Under IdeS digestion, a mAb reduces to three 25 kDa-sized fragments, Fd', Fc, and light chain (LC) that can easily be analyzed using LC (Figure 1).2
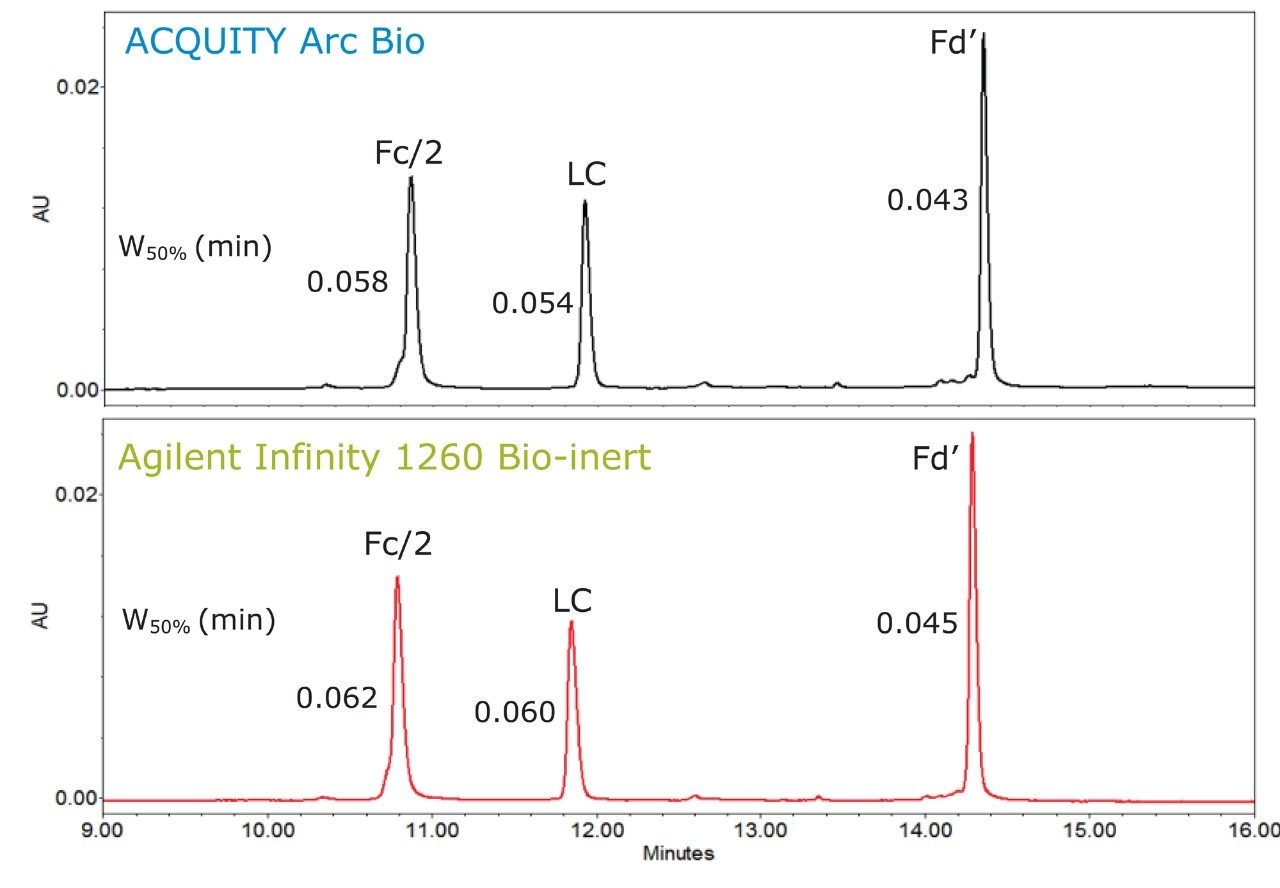
To assess the transferability of the separation, the mAb Subunit Standards were analyzed on two biocompatible LC systems, an ACQUITY Arc Bio System using flow path 1 and an Agilent 1260 Infinity Bio-inert System. While a number of factors can impact method transfer, the systems selected had similar characteristics such as, dwell volume and extra-column volume. Therefore, comparable chromatographic profiles were observed between the two systems (Figure 2).
As mentioned, method transfer across different LC systems can be affected by extra-column volume.3 Extra-column volume is a measure of the band broadening in LC systems and is related to the volume from the injector to the detector. It impacts peak width, resolution, and overall efficiency of a separation. The extra-column volume for both systems were found to be similar, with the ACQUITY Arc Bio producing a value of 19 µL while the Agilent 1260 Infinity Bio-inert gave a value of 24 µL, both at 4σ. Given these values, it is not surprising to see that the peaks have similar peak width, albeit the values observed on the ACQUITY Arc Bio System were slightly lower (Figure 2).
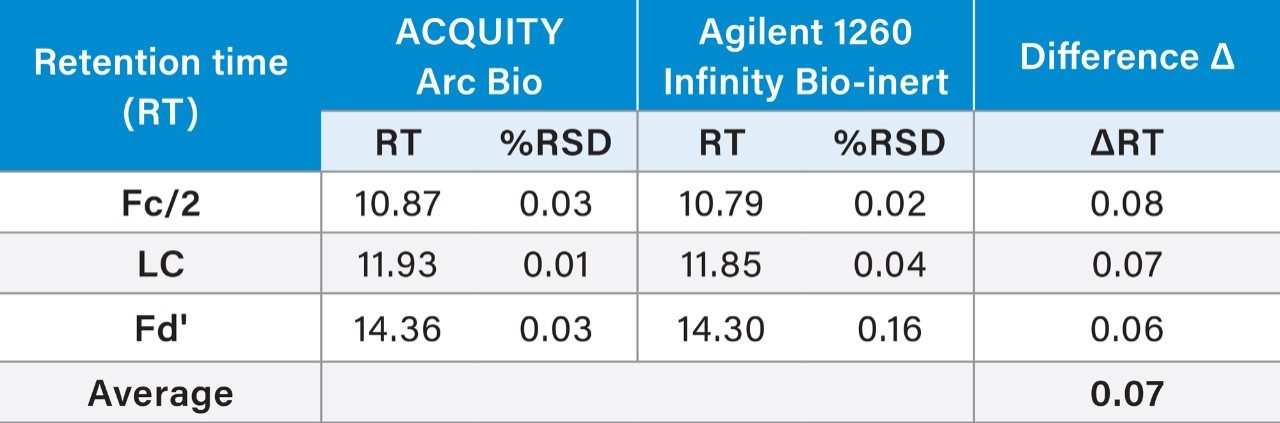
Another instrument characteristic that can impact LC method transfer is dwell volume. Dwell volume, the volume in which the programmed gradient reaches the head of the column, typically affects the retention times of a gradient separation. Following a specific protocol,3 the measured dwell volume of the ACQUITY Arc Bio System was 1.32 mL for flow path 1 and 1.01 mL for flow path 2. The dwell volume measured for the Agilent Infinity 1260 Bio-inert System was 1.57 mL. The dwell volume of flow path 1 on the ACQUITY Arc Bio was closer to that of the Agilent system, thus it was selected for the RP method transfer.
The results show the comparability of both the average retention times (RT) along with %RSD (Table 1). The average shift in retention time was 0.07 min, resulting <1% RT difference across the two systems. If dwell volume were the only factor to impact the retention time, we would have expected the RT shift between these two systems is 0.26 min (0.25 mL (1.57 mL–1.32 mL)/0.96 mL/min). However, other factors also play a role in the retention time of the separation, such as pump mixing behavior, mobile phase properties, system pressure, column temperature control, etc. These factors are more pronounced when method is transferred among LC systems from different vendors.
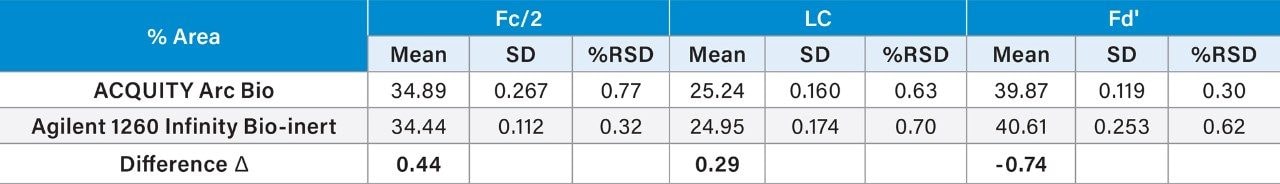
In addition to the retention time, peak area percent (% area) and repeatability for the mAb subunits were also analyzed (Table 2). Comparison of the % area showed similar values across both systems with a difference (Δ) of <1% for all three subunits. Furthermore, the repeatability, as measured by %RSD was <1% for triplicate analysis. Thus, the quantification of % area of the mAb subunits is reproducible across these two systems.
During LC method transfer, retention time shifts are commonly observed. Many laboratories require no adjustments to a method, particularly for validated methods. According to regulatory guidelines, the duration of an initial isocratic hold, and/or dwell volume adjustments are allowed to address differences in dwell volumes among LC systems.4 Manually entering into a gradient table to adjust for dwell volume requires additional time and efforts. ACQUITY Arc Bio Systems are equipped with Arc Multi-flow path Technology to allow users to select between two flow paths with different dwell volumes. The Gradient SmartStart feature allows users to fine-tune retention time based on the observed retention time shifts. This feature adjusts the time between the start of the gradient and the point of injection. The adjustment uses either time or volume for intuitive adjustment.
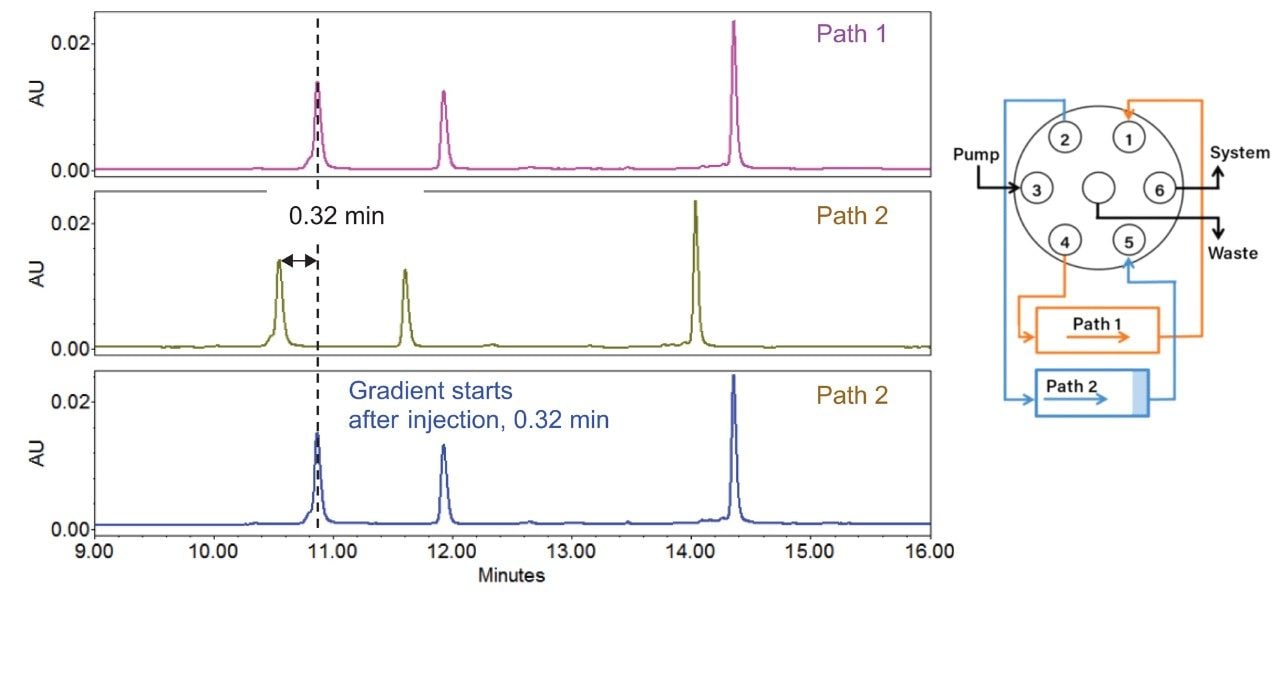
To demonstrate the use of Multi-flow path Technology and Gradient SmartStart, the same RP analysis was performed on both the path 1 (Figure 3A) and path 2 (Figure 3B) on the ACQUITY Arc Bio System. As previously described, the measured dwell volume difference between path 1 and path 2 was 0.31 mL. Considering the flow rate of 0.96 mL/min, a retention time shift of 0.32 min (0.31 mL/0.96 mL/min) should be observed. In fact, the results matched the difference in dwell volume. In this case, dwell volume was the major factor to impact retention since all other instrument characteristics remained the same.
Given the shift in retention time, Gradient SmartStart can be used to fine tune the retention. Based on the previous results, in the instrument control software, 0.32 min after injection was enabled with selection of path 2. The resulting chromatogram (Figure 3C) produced the same retention as in path 1 (Figure 3A). Therefore, by utilizing both Multi-flow path Technology and Gradient SmartStart, the ACQUITY Arc Bio System can address retention time shifts for dwell volume differences across systems, without the need to change the gradient table.
Chromatographic system characteristics, including extra-column dispersion and dwell volume, impact chromatographic performance. This can be particularly apparent during method transfer. In this study, a RP method of separating mAb subunits was transferred across an Agilent 1260 Infinity Bio-inert System and an ACQUITY Arc Bio System. Flow path 1 on the ACQUITY Arc Bio System was selected to match the dwell volume on Agilent system. The difference in retention times across the systems were <0.1 min. The repeatability of retention time was within 0.2% and that for peak area% for three mAb subunits was <1%. The peak width for all the three mAb subunits is comparable since the system extra-column volume are similar for both systems. The RP method is replicated from the Agilent 1260 Infinity Bio-inert System to the ACQUITY Arc Bio System. Multi-flow path Technology built in the ACQUITY Arc Bio System contributes to the successful method transfer in this case study. Along with Gradient SmartStart, the ACQUITY Arc Bio System is enabled to accept and replicate methods from a variety of LC systems.
720006350, August 2018