This application note demonstrates the benefits of the unique stationary phase of BioResolve RP mAb Polyphenyl Columns. The unique Polyphenyl bonded phase of the BioResolve RP mAb Column was developed to make it possible to elicit more information regarding the microheterogeneity of therapeutic proteins by improving the overall quality of RPLC mAb and mAb subunit separations. Designing the bonded phase to have the distinctive benefits of a high-coverage, phenyl-based chemistry has resulted in noteworthy protein separation capabilities. The BioResolve RP mAb Polyphenyl Column provides favorable selectivity versus other stationary phases while producing higher peak capacities for a variety of ion pairing conditions. Moreover, the Polyphenyl chemistry lends excellent acid stability by the nature of its high coverage bonding, which also aids in reducing carryover and allows for separations using lower temperatures and lower ion pairing strength. In all, the novel bonded phase of the BioResolve RP mAb Polyphenyl Column provides chromatographers with an opportunity to achieve higher resolution and to explore the use of lower temperatures and more MS-friendly conditions.
The inherent complexity and microheterogeneity of proteins requires that they be extensively characterized if they are to be used as therapeutic modalities, as is the case with many monoclonal antibodies (mAbs). High performance liquid chromatography is the most widely used technique for detecting the molecular variability of protein and peptide therapeutics. Reversed-phase liquid chromatography (RPLC) is particularly useful because of its high resolution and amenability to mass spectrometry detection. However, it can be a challenge to achieve efficient separations of proteins due to their size and complexity. RPLC separations of proteins are strongly dependent on the surface chemistry of the particle, where undesirable interactions with protein sites may cause peak broadening and high carryover.1
In recent years, new bonded phases have been proposed as alternatives to common alkyl bondings (e.g., C4 and C8) for RPLC separations of proteins. In particular, phenyl chemistries show interesting selectivity in resolving variants of monoclonal antibodies and antibody fragments due to having a larger ligand surface for protein contact and the potential for pi-pi interactions.2 Another chemistry frequently used for protein separations is polydivinylbenzene (DVB), a highly hydrophobic material that is free of silanol activity, which can be quite pronounced for bonded silicas. With these two chemistries being considered, it was hypothesized that a stationary phase combining an optimized solid-core silica particle substrate3 with a surface chemistry reminiscent of a DVB polymer could result in very promising protein chromatography.
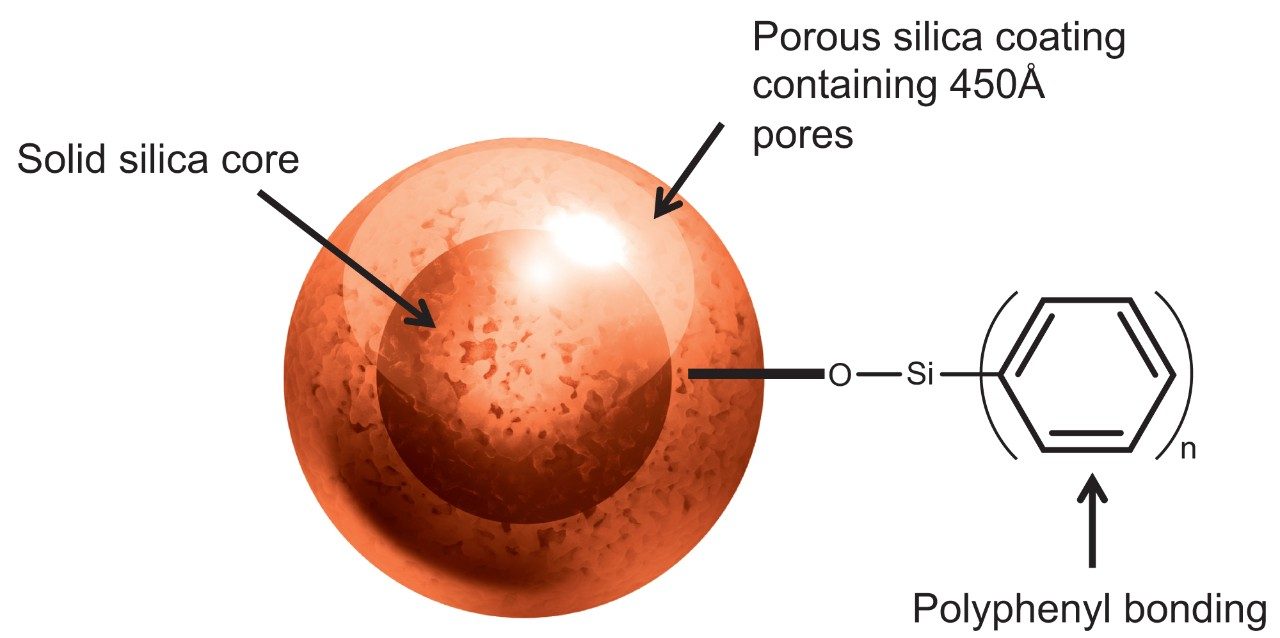
To this end, a novel phenyl surface chemistry has been developed using a multistep synthesis process to yield a uniquely high coverage bonded phase on the optimized solid-core, 450Å, silica particle (Figure 1). This Polyphenyl bonding is both high in coverage (up to 6 µmol phenyl moiety/m2) and comprised of rigidly constrained carbons. The sum of these properties is believed to limit silanol interactions by extensively masking the silica surface. As shown, we believe this facilitates more discrete protein-to-ligand desorption at lower temperatures by minimizing the conformational heterogeneity of protein adsorption and improving resolving power by being highly retentive.
This application note will demonstrate the benefits of the unique stationary phase of BioResolve RP mAb Polyphenyl Columns, including high effective peak capacities, enhanced selectivity, low carryover, excellent acid stability, and lessened temperature and ion pairing dependence versus C4 and DVB stationary phases.
Reduced, IdeS digested NIST mAb was acquired in the form of the Waters mAb Subunit Standard (p/n: 186008927). Each vial of this standard contains approximately 25 µg of subunits and was reconstituted in 100 µL of 0.1% (v/v) formic acid (FA) in water.
Infliximab (Remicade), 10 mg/mL, was diluted into 13 mM sodium phosphate buffer (pH 7.1) and incubated at a concentration of 2 mg/mL with IdeS for 30 minutes at 37 °C at a ratio of 1 µg of infliximab per 1 unit of IdeS. The resulting preparation of subunits was stored at -80 °C until analyzed.
A lyophilized pellet of Intact mAb Mass Check Standard (1 mg, p/n: 186006552) was reconstituted in 500 µL (2 mg/mL) of 0.1% (v/v) aqueous formic acid.
A stock solution containing 25 µg/mL thiourea, 525 µg/mL aniline, 50 µg/mL methylparaben, and 300 µg/mL phenol was prepared in water.
All columns were conditioned using the Intact mAb Mass Check Standard according to the guidelines provided in the BioResolve RP mAb Polyphenyl Column Care and Use Manual (p/n: 720006027EN).
|
Instrument: |
ACQUITY UPLC H-Class Bio |
|
Data management: |
MassLynx v4.1, UNIFI v1.8 |
|
Columns: |
BioResolve RP mAb Polyphenyl, 450Å, 2.7 μm, 2.1 x 50 mm (p/n: 176004156; includes column, Intact mAb and mAb Subunit Standards) |
|
|
Other columns, 2.1 x 50 mm |
|
Mobile phase A: |
0.1% FA or TFA (v/v) in water |
|
Mobile phase B: |
0.1% FA or TFA (v/v) in acetonitrile |
|
Gradient: |
15–55% B in 20 min with a repeated gradient when measuring intact mAb carryover effects |
|
Flow rate: |
0.2 mL/min |
|
Column temp.: |
80 °C |
|
Detection (UV): |
280 nm |
|
Mass load: |
1 μg |
Effective peak capacity (Pc*) was calculated from Eq 1.
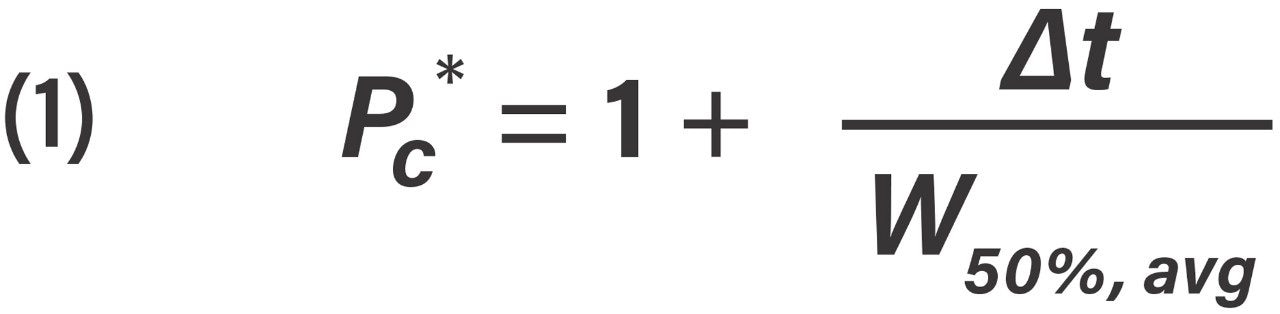
Effective peak capacity equation, where Δt is the difference in retention times for the first eluting peak and the last eluting peak (Fc/2 of infliximab and Fd' of NIST mAb subunits), and W50%, avg is the average peak width at half height for the analyte(s) under investigation.
Percent carryover was calculated from Eq 2.
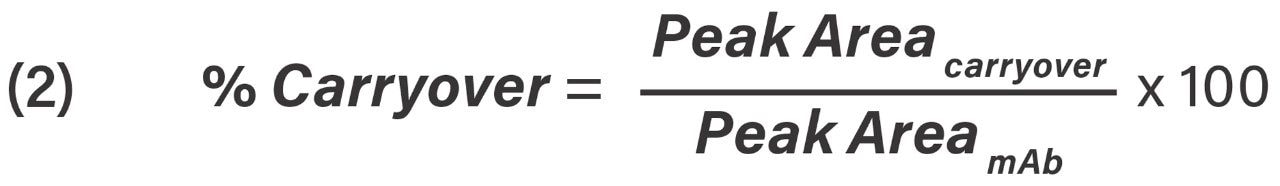
Percent carryover equation, where Peak Areacarryover is the peak area of the intact mAb from the double gradient and Peak AreamAb is the peak area of the intact mAb from the first gradient.
|
Instrument: |
ACQUITY UPLC H-Class Bio |
|
Data management: |
Empower 3 FR2 |
|
Columns: |
BioResolve RP mAb Polyphenyl, 450Å, 2.7 μm, 2.1 x 50 mm (p/n: 176004156; includes column, Intact mAb and mAb Subunit Standards) |
|
|
Other columns, 2.1 x 50 mm |
|
Mobile phase: |
0.5% TFA in water (v/v) |
|
Elution mode: |
Isocratic |
|
Flow rate: |
1.40 mL/min |
|
Column temp.: |
60 °C |
|
Detection (UV): |
254 nm |
|
Injection volume: |
2 μL |
Retention factor k' for methylparaben was calculated from Eq 3.
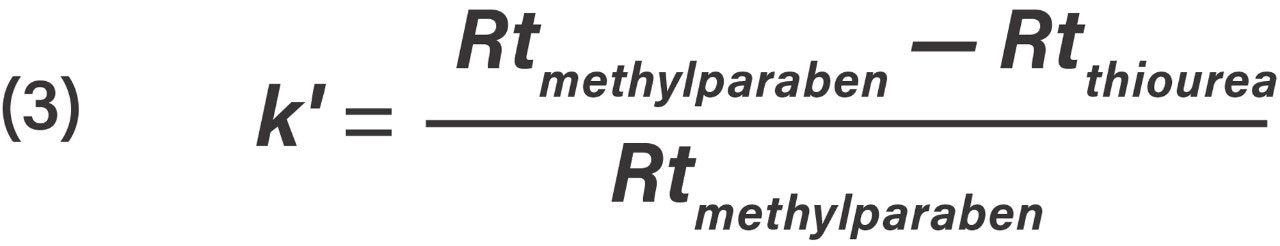
Retention factor equation, where Rtmethylparaben is the retention time of methylparaben and Rtthiourea is the retention time of thiourea.
Percent change in k' for methylparaben was calculated from Eq 4.
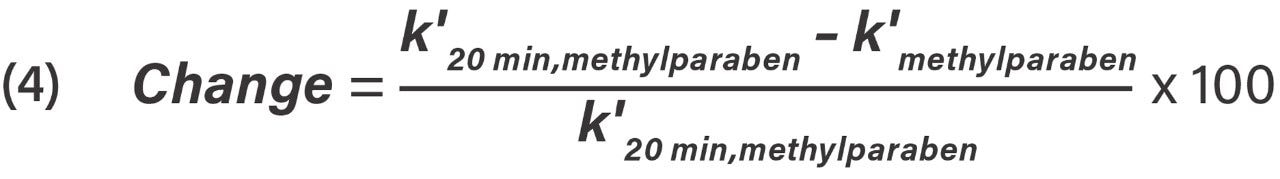
Retention factor equation, where k'20 min, methylparaben is the retention factor of methylparaben at 20 minutes exposure time and k'methylparaben is the retention factor of methylparaben at an exposure time past 20 minutes.
|
Instrument: |
ACQUITY UPLC H-Class Bio |
|
Data management: |
MassLynx v4.1, UNIFI v1.8 |
|
Columns: |
BioResolve RP mAb Polyphenyl, 450Å, 2.7 μm, 2.1 x 100 mm (p/n: 176004156; includes column, Intact mAb and mAb Subunit Standards) |
|
Mobile phase A: |
0.1% TFA (v/v) in water |
|
Mobile phase B: |
0.1% TFA (v/v) in acetonitrile |
|
Gradient: |
15–55% B in 10 min |
|
Flow rate: |
0.8 mL/min |
|
Column temp.: |
80 °C |
|
Detection (UV): |
280 nm |
|
Mass load: |
1 μg |
|
Instrument: |
ACQUITY UPLC H-Class Bio |
|
Data management: |
MassLynx v4.1, UNIFI v1.8 |
|
Columns: |
BioResolve RP mAb Polyphenyl, 450Å, 2.7 μm, 2.1 x 50 mm (p/n: 176004156; includes column, Intact mAb and mAb Subunit standards |
|
|
Other columns, 2.1 x 50 mm |
|
Mobile phase A: |
0.1% FA, 0.02% TFA/0.08% FA, or 0.1% TFA (v/v) in water |
|
Mobile phase B: |
0.1% FA, 0.02% TFA/0.08% FA, or 0.1% TFA (v/v) in acetonitrile |
|
Gradient: |
15–55% B in 10 min |
|
Flow rate: |
0.4 mL/min |
|
Column temp.: |
30, 40, 50, 60, 70, 80, or 90 °C |
|
Detection (UV): |
280 nm |
|
Mass load: |
1 μg |
Effective peak capacity (Pc*) was calculated from Eq 1, where in this case Δt is the retention time difference between the Fc/2 and the Fd' components of solely the mAb Subunit Standard.
|
Instrument: |
ACQUITY UPLC H-Class Bio |
|
Data management: |
MassLynx v4.1, UNIFI v1.8 |
|
Columns: |
BioResolve RP mAb Polyphenyl, 450Å, 2.7 μm, 2.1 x 150 mm (p/n: 176004156; includes column, Intact mAb and mAb Subunit Standards) |
|
Mobile phase A: |
0.05% or 0.1% TFA (v/v) in water |
|
Mobile phase B: |
0.05% or 0.1% TFA (v/v) in acetonitrile |
|
Gradient: |
15–55% B in 20 min |
|
Flow rate: |
0.6 mL/min |
|
Column temp.: |
65 or 80 °C |
|
Detection (UV): |
280 nm |
|
Mass load: |
1 μg |
Effective peak capacity (Pc*) was calculated from Eq 1, where in this case Δt is the retention time difference between the Fc/2 and the Fd' components of solely the mAb Subunit Standard.
To evaluate the chromatographic performance of the Polyphenyl bonding, BioResolve RP mAb Polyphenyl Columns were compared to several leading columns based on silica, hybrid, and DVB stationary phase base particles. The peak capacities of the columns were determined using multiple mAb based species ranging from 25 to 150 kDa in molecular weight. These analytes were separated using both formic acid (FA) and trifluoroacetic acid (TFA) modified mobile phases. The separations were performed on 2.1 mm I.D. columns using an optimized mass load of 1 µg per analyte. Since surface chemistry affects retentivity, we calculated the effective peak capacities (see Eq 1) for accurate representations of resolving power.
Figure 2A shows the results obtained using 0.1% TFA modified mobile phases. The BioResolve RP mAb Polyphenyl Column produced an average effective peak capacity from all three mAb test samples (Pc,Avg*), that is, 13 to 50% higher than those of the other columns. Figure 2B shows that the BioResolve RP mAb Polyphenyl Column gave the highest effective peak capacity for each analyte. Interestingly, the performance of the ACQUITY UPLC Protein BEH C4, 300Å Column is also quite compelling for the lower molecular weight analytes (the mAb subunits) under these conditions. From these chromatograms, it can be seen that the BioResolve RP mAb Polyphenyl Column gives partial resolution of several low abundant species, such as the lysine variant at the front of the mAb subunit Fc/2 peak.
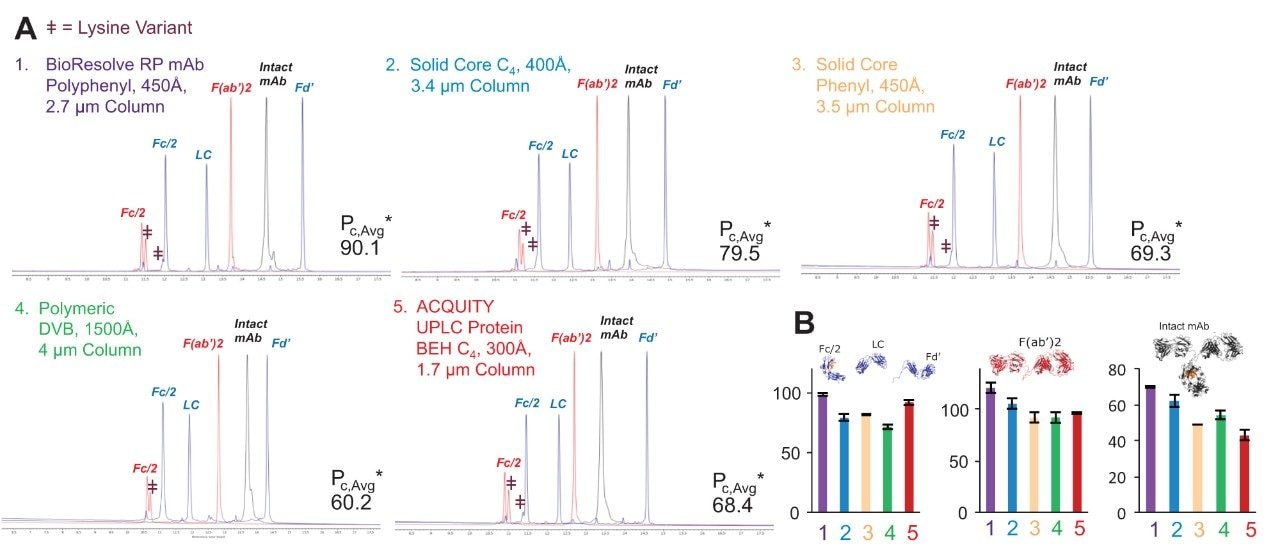
To assess their amenability to more MS-compatible conditions, the columns were also tested using 0.1% FA modified mobile phases. Because of its weak ion pairing properties, FA can be very challenging to use without compromising the resolution or reproducibility of a separation. Nevertheless, it is frequently preferred for LC-MS analyses because it gives high detection sensitivity. Figure 3A compares the chromatograms for the same three mAb-based samples using this additive. The BioResolve RP mAb Polyphenyl Column gives the best performance under these conditions, as is visually indicated by the increased resolution of all Fc/2 lysine variants as well as the overall reduction in peak tailing. As for the results using TFA, the BioResolve Column produced the highest average, effective peak capacity, as well as the highest, effective peak capacities for each individual protein species (Figure 3B).
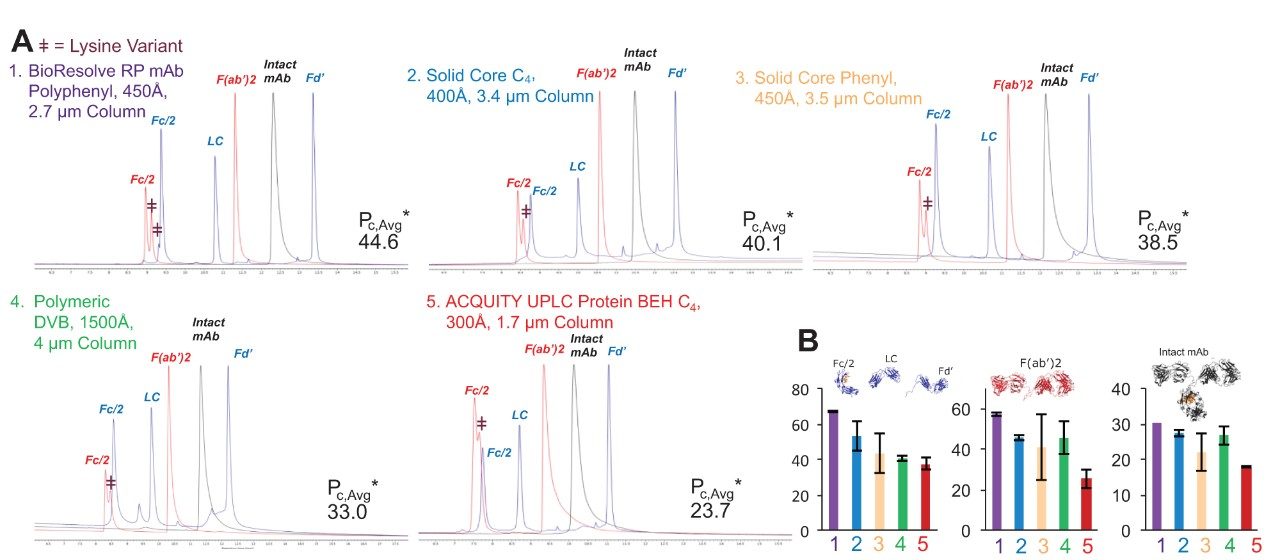
We attribute the performance of the BioResolve RP mAb Polyphenyl Column to its optimized wide-pore, solid-core particle and Polyphenyl bonding. The high coverage of rigidly constrained carbons and sterically bulky phenyl ligands might limit the degrees of freedom for the conformational states of protein-to-sorbent interactions and attenuate protein-sorbent secondary interactions.
Resolution depends not only on peak sharpness but also on retention and selectivity. In addition to the high peak capacities of BioResolve RP mAb Polyphenyl Columns, they also show advantageous selectivity. Figure 4 compares separations of the Waters Intact mAb Mass Check Standard obtained with the BioResolve RP mAb Polyphenyl Column versus a C4-bonded prototype based on the same particle. It is clear that the Polyphenyl column resolves variants from the standard considerably better than does its C4 analog. This observation is consistent with previous literature concerning phenyl-bonded chemistries.4

In addition to optimizing resolving power, it is critical for injection-to-injection carryover to be minimal. Carryover, or memory effects, can occur when a protein desorbs very slowly from a column. It must be minimized to avoid interferences between consecutive sample injections and inaccurate quantitative results from each analysis. To quantify carryover, a BioResolve RP mAb Polyphenyl Column and four leading columns (all 2.1 x 50 mm) were subjected to 1 µg mass loads of Waters Intact mAb Mass Check Standard and an initial gradient followed by a second, repeated gradient with no injection. These studies were carried out using both TFA- and FA-modified mobile phases.
Figure 5A shows the intact mAb peak in the initial gradient while Figure 5B displays the retention window corresponding to the repeated gradient (at a 50× magnification), using a FA-modified mobile phase. The quantitative measurements in Figure 5C show that the benchmark columns gave higher levels of carryover compared to the BioResolve RP mAb Polyphenyl Column, which gave no detectable carryover at 280 nm detection under these identical instrument, sample, eluents, and gradient elution conditions. When using a TFA-modified mobile phase (Figure 5D), the trend remained the same – the BioResolve RP mAb Polyphenyl Column exhibited lower carryover than the benchmark columns. These differences in carryover demonstrate that the stationary phase plays a primary role in maximizing the rate of protein desorption and thus minimizing carryover effects.
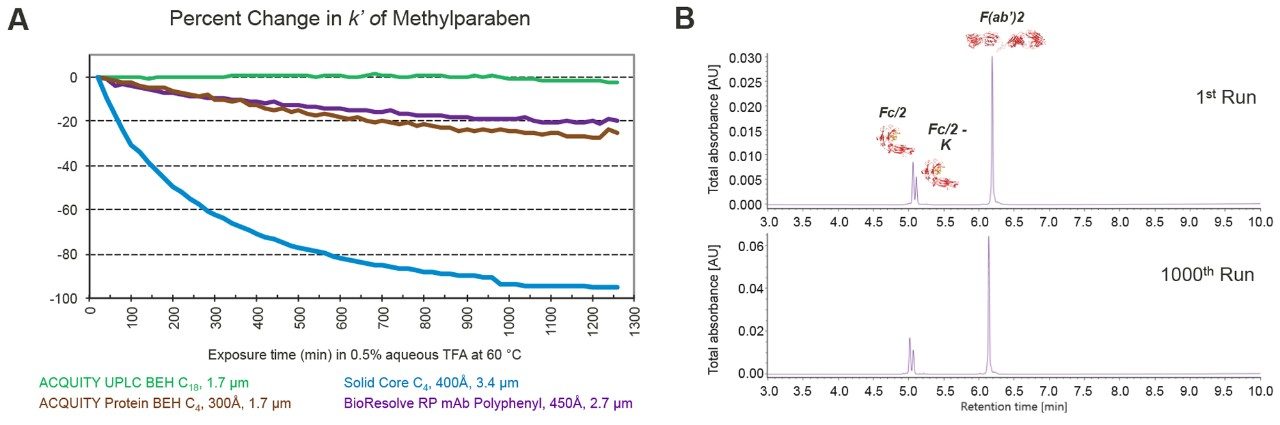
Reversed-phase protein separations are usually performed using acidic, ion-pairing mobile phases at high temperatures. It is well known that these aggressive conditions can catalyze the hydrolysis of bonded phases, resulting in deterioration in chromatographic performance. Acid stability can be readily monitored under accelerated test conditions (e.g, using 0.5% TFA) as a decrease in analyte retentivity.5 In one test method, a column’s retention factor for methyl paraben is measured as a function of time exposed to a 0.5% aqueous TFA mobile phase at 60 °C. We used this test to compare the acid stability of a BioResolve RP mAb Polyphenyl Column to that of three benchmark columns. As seen in the graph in Figure 6A, the BioResolve Column demonstrated good stability that is on par with an ACQUITY UPLC Protein BEH C4, 300Å Column, losing only 20% of its retentivity after approximately 21 hours of exposure to these harsh conditions. This result far outperforms an n-C4 solid core column, where the retention of methyl paraben virtually disappears over the same amount of time, indicating extensive bonded phase loss. Factors that impact resistance to acid hydrolysis include ligand chain length, steric bulk, bonding density, and functionality (attachment by means of mono-, di-, or trifunctional silanes).5 The Polyphenyl bonding was designed to produce a high coverage of phenyl groups, forming a highly hydrophobic surface that minimizes the rate of hydrolysis.
Testing a BioResolve RP mAb Polyphenyl Column under typical conditions showed very reproducible separations over a week of continuous use. In this test, the most acidic of the commonly employed mobile phases (0.1% TFA) was used along with a column temperature of 80 °C. The results (Figure 6B) showed little change in a separation of infliximab subunits over 1000 runs. A quantitative comparison of chromatographic performance supports this observation. Less than a 1% change in retention time was observed for all subunits when comparing the first injection to the last, and in terms of peak widths, the Fc/2 subunit peaks actually became subtly sharper, with improvements of 2 and 3% respectively. Meanwhile, the peak width of the larger F(ab)'2 fragment increased by only 4%. These results demonstrate that the Polyphenyl bonding provides excellent hydrolytic stability, which in turn enables an analyst to more reliably reproduce separations on a BioResolve RP mAb Polyphenyl Column over long periods of use.
Another consideration in designing bonded phase chemistries for RPLC separations of proteins is their dependence on elevated separation temperatures. Temperatures up to 80 to 90 °C have consistently been required for reversed-phase separations of mAbs. However, when combined with strongly acidic conditions, such temperatures, can cause on-column protein degradation.1 Accordingly, it is beneficial to reduce the temperature of RPLC separations of proteins whenever possible.
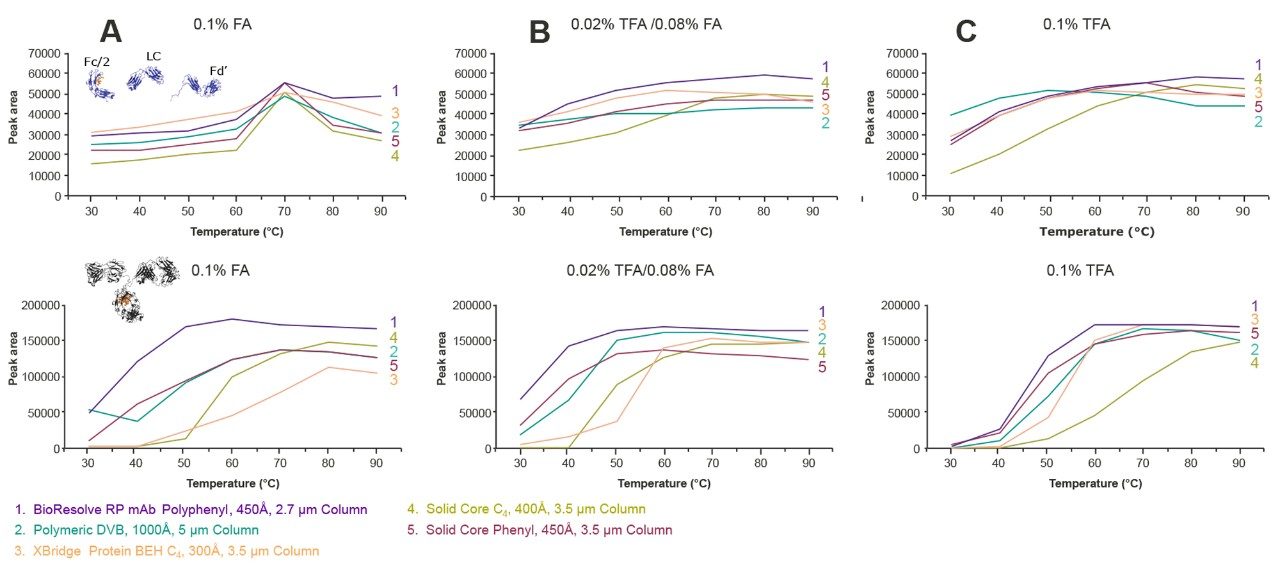
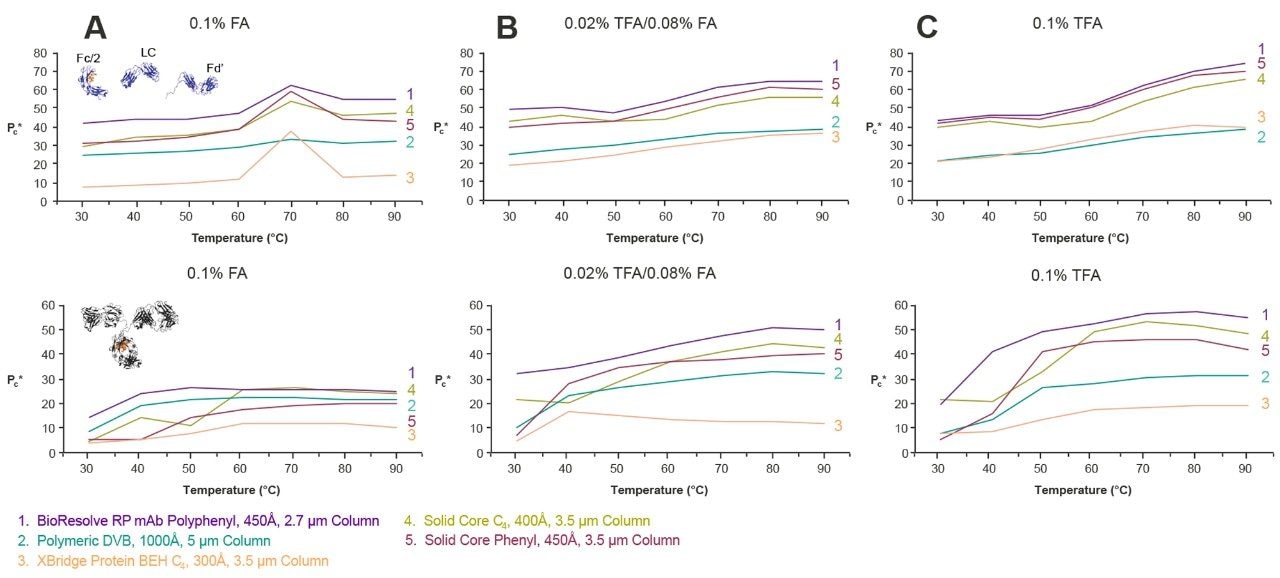
There are two key aspects of a protein separation that vary with temperature: recovery and resolution. These responses were determined for the mAb Subunit Standard and the Intact mAb Mass Check Standard as a function of temperature using three different ion pairing conditions (0.1% FA, 0.02% TFA/0.08% FA, and 0.1% TFA). Peak areas were measured as a surrogate for recovery and effective peak capacity was used as a representation of resolution. Tests were carried out for a BioResolve RP mAb Polyphenyl Column as well as for the four benchmark columns.
The results of these studies are shown in Figures 7 and 8. The peak area results, shown in Figure 7, indicate that recovery can vary to a surprising degree when ion pairing conditions are changed, at least for the two sets of analytes investigated in this study. The strongest ion pairing condition, 0.1% TFA, resulted in the highest protein peak areas and peak capacities, so long as very high temperatures were employed. Nonetheless, the use of 0.02% TFA/0.08% FA also produced comparatively high peak areas and peak capacities. In fact, at lower temperatures, the lower concentration of TFA proved to be advantageous. However, Figures 7 and 8 suggest that protein separations using 0.1% FA might be less tolerant of reduced temperatures (like 0.1% TFA), particularly for the mAb subunits tested here at temperatures below 70 °C. In all, these examples show that the temperature dependence of RPLC separations of proteins is intimately tied to ion pairing.
For all three ion pairing conditions, the BioResolve RP mAb Polyphenyl Column afforded the highest recoveries out of the cohort of columns investigated, with the exception of the polymeric DVB column used with 0.1% TFA at low temperatures for the mAb subunits. As shown in Figure 8, the BioResolve RP mAb Polyphenyl Column also exhibits the kinetic advantages inherent to solid-core particle technology, giving the highest peak capacities for all ion pairing conditions over the temperature range investigated. BioResolve RP mAb Polyphenyl Columns thus offer the potential to use temperatures ranging from 50 to 70 °C, while also employing more MS-friendly, ion pairing conditions. This allows both sample and column degradation to be reduced. However, due to the complexity of proteins, it is expected that there will be caveats to the use of these alternative conditions, and that the results will be sample dependent.
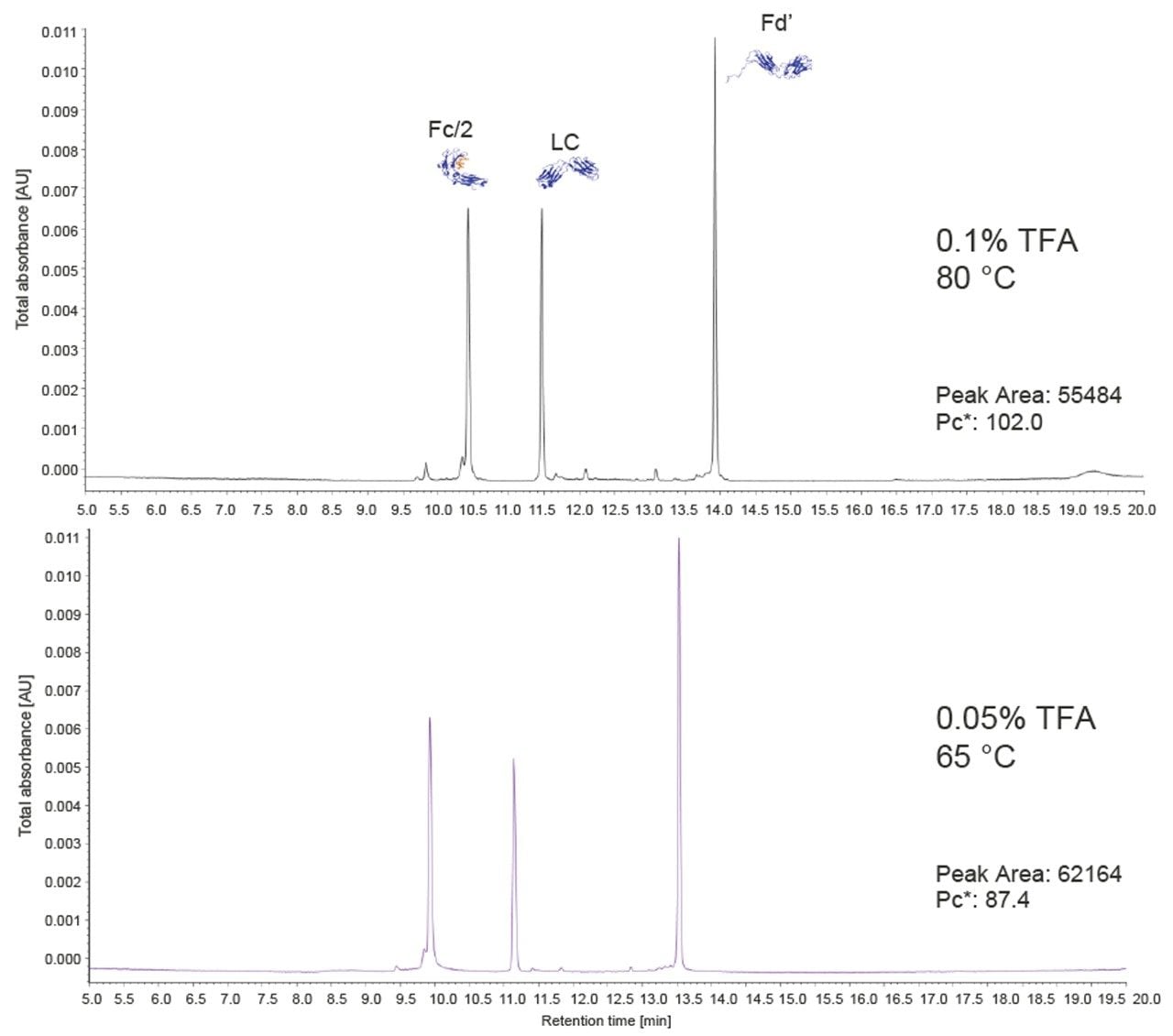
The distinctive properties of the Polyphenyl bonding can be appreciated through an example that demonstrates the development of a high-resolution subunit separation using a reduced temperature and lowered concentration of ion pairing additive. Figure 9 depicts the separation of the mAb Subunit Standard using a 2.1 x 150 mm column under two different conditions: (1) with a temperature of 65 °C and 0.05% TFA-modified mobile phases, and (2) at 80 °C with 0.1% TFA. All three subunit peaks (Fc/2, LC, and Fd') are baseline resolved under both conditions. Looking closely, small peaks from low abundant species can also be seen in both chromatograms. These peaks are noticeably smaller in the 65 °C/0.05% TFA separation. It has been observed elsewhere that lower temperatures and reduced concentrations of strong acid reduce the rate of acid catalyzed backbone cleavage that produces these degradants.6,7 In particular, the Fd' pre-peak likely corresponds to an artifactual internal cleavage between residues D88 and P89.6 Lowering the TFA concentration and temperature markedly reduced this degradation. Using a BioResolve RP mAb Polyphenyl Column can help avoid these degradation effects by allowing the use of lower temperatures and reduced concentrations of ion pairing species.
Moreover, being able to use lower concentrations of TFA is of benefit to MS detection. Holistically speaking, the BioResolve RP mAb Polyphenyl Column facilitates obtaining very high resolution separations with more MS and sample friendly conditions such that higher fidelity data can be generated. An example of this can be seen in Waters technology brief 720006199EN.8
The unique Polyphenyl bonded phase of the BioResolve RP mAb Column was developed to make it possible to elicit more information regarding the microheterogeneity of therapeutic proteins by improving the overall quality of RPLC mAb and mAb subunit separations. Designing the bonded phase to have the distinctive benefits of a high-coverage, phenyl-based chemistry has resulted in noteworthy protein separation capabilities. The BioResolve RP mAb Polyphenyl Column provides favorable selectivity versus other stationary phases while producing higher peak capacities for a variety of ion pairing conditions. Moreover, the Polyphenyl chemistry lends excellent acid stability by the nature of its high coverage bonding, which also aids in reducing carryover and allows for separations using lower temperatures and lower ion pairing strength. In all, the novel bonded phase of the BioResolve RP mAb Polyphenyl Column provides chromatographers with an opportunity to achieve higher resolution and to explore the use of lower temperatures and more MS-friendly conditions.
720006169, March 2018