
This is an Application Brief and does not contain a detailed Experimental section.
This technical brief shows the comparison of ionization of a selection of pharmaceutical compounds by electrospray (ESI) and impactor ionization (UniSpray) as well as their robustness in human serum.
Ionization plays a critical role in analysis by mass spectrometry with multiple ionization techniques available. Here we describe an impactor ionization source and its application to the analysis of pharmaceutical drugs.
Impactor ionization is the formation of ions by directing a heated nebulized spray of liquid onto a surface with an applied voltage.¹ The spray is aimed off center and on impact, the ions flow downstream in a path that follows the curvature of the surface (in this case a pin), called the Coandă effect (Figure 1). Commonly used electrospray ionization involves a heated high velocity spray from a charged capillary. These two techniques have similar effects of ionization, producing predominantly [M+H]+ (or [M-H]-) ions, yet their mechanisms appear to be different. This work shows the comparison of ionization of a selection of pharmaceutical compounds by electrospray (ESI) and impactor ionization (UniSpray) as well as their robustness in human serum.

|
LC system: |
ACQUITY UPLC I-Class FTN |
|
Vials: |
Waters TruView LC-MS Certified, Total Recovery Vial [P/N 186005663CV] |
|
Column: |
ACQUITY BEH C18 , 2.1 x 50 mm, 1.7 μm [P/N 186002350] |
|
Column temp.: |
30 °C |
|
Sample temp.: |
5 °C |
|
Injection volume: |
3 μL |
|
Flow rate: |
0.600 mL/min |
|
Mobile phase A: |
H2O, 0.1% formic acid |
|
Mobile phase B: |
Acetonitrile, 0.1% formic acid |
|
Gradient: |
1 to 95 %B over two minutes, hold for 0.5 minutes |
|
MS system: |
Xevo TQ-S Mass Spectrometer |
|
Acquisition mode: |
MRM |
|
Ionization type: |
Electrospray and UniSpray |
|
Ionization mode: |
+/- |
|
Capillary voltage: |
1 kV/2 kV POS/NEG |
|
Desolvation temperature: |
550 °C |
|
Desolvation flow: |
1100 L/hr |
Compound libraries with molecular weights ranging from 151 to 824 were purchased from Enzo Life Sciences. The standards were diluted to 100 nM and 1 nM, or 0.1 nM with 30:70:0.1 ACN/water/formic acid for high-throughput compound optimization of [M+H]+ or [M-H]- by QuanOptimize followed by injection on column. Plasma robustness studies were performed by protein precipitation with ACN at a 3:1 ratio, vortex mixed, and then centrifuged at 16.1 x g for 10 minutes. The supernatant was taken and standards were spiked to 1 nM before injection. An ACQUITY UPLC I-Class System with a BEH C18 Column (2.1 x 50 mm) and mobile phases of water and ACN with 0.1% formic acid was coupled to a Xevo TQ-S Mass Spectrometer equipped with ESI and UniSpray ionization sources. Data was collected in both positive and negative ionization modes and processed using MassLynx v.4.1. All results were calculated on chromatographic peak area.
The UniSpray source was tuned using 50% mobile phases A and B at analytical flow combined with 10 µL/min of 10 ng/mL verapamil. The capillary protrusion was first optimized for stable spray followed by positional adjustment of the capillary with respect to the pin. The highest signal is seen when the spray is directed to the right of center on the pin, as shown in Figure 1. It should be noted that the following data is from a single tune position. Custom tuning on specific target compounds may yield better sensitivity for compounds that initially showed a poor response compared to ESI.
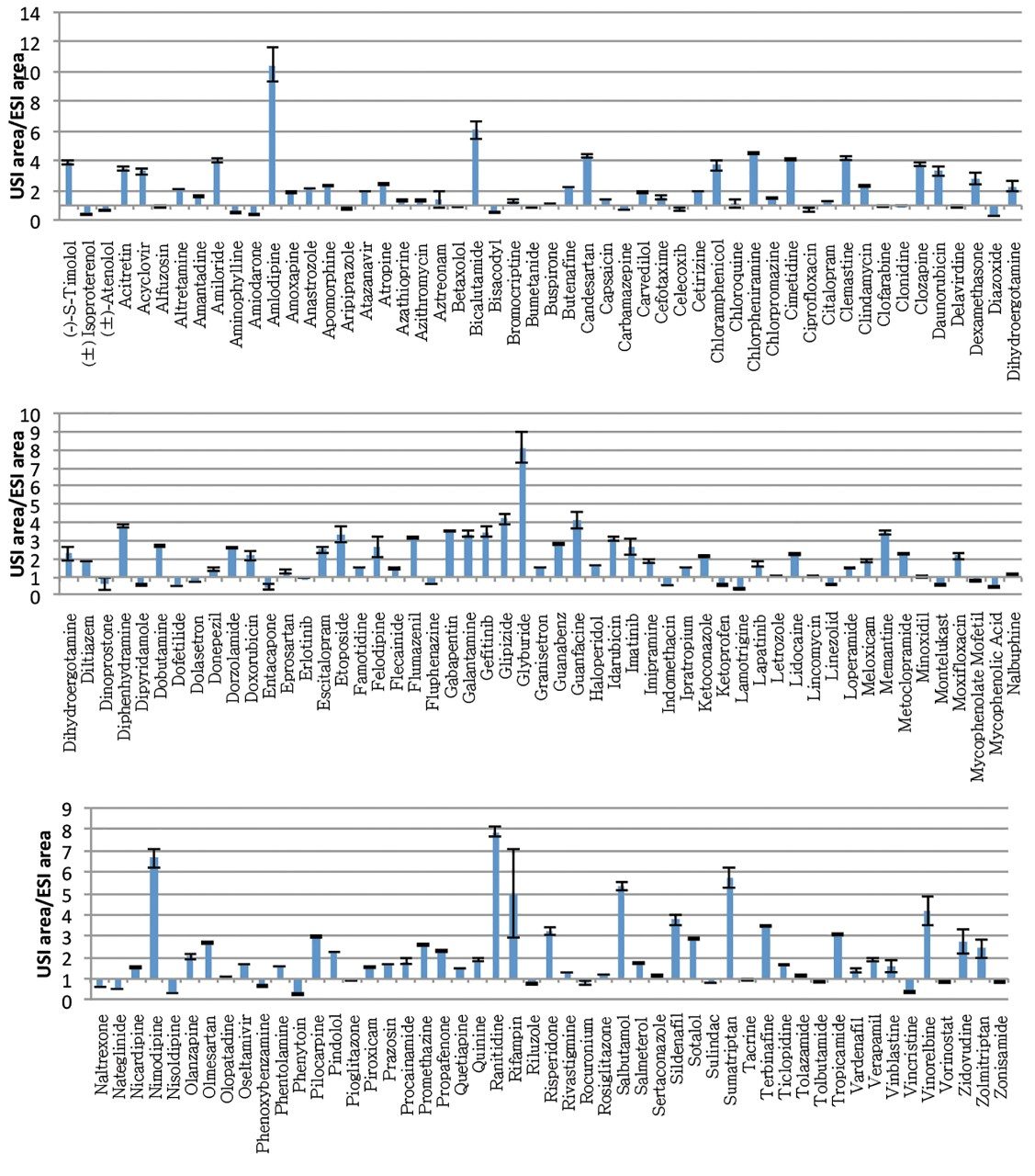
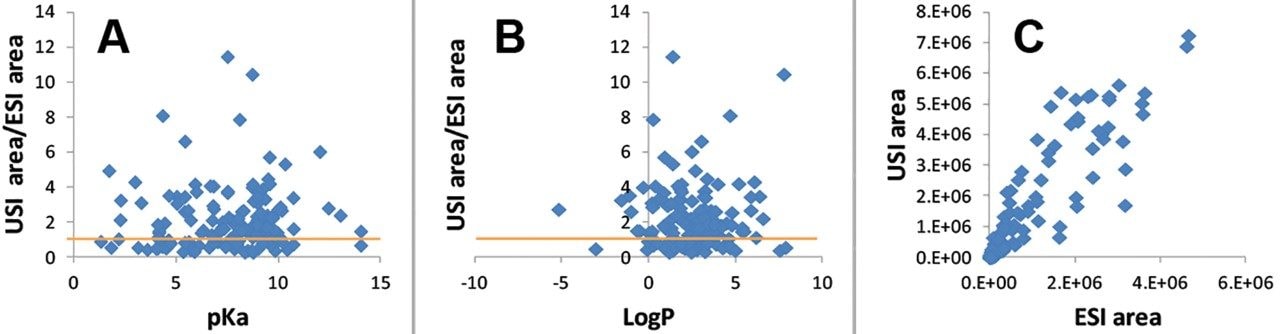
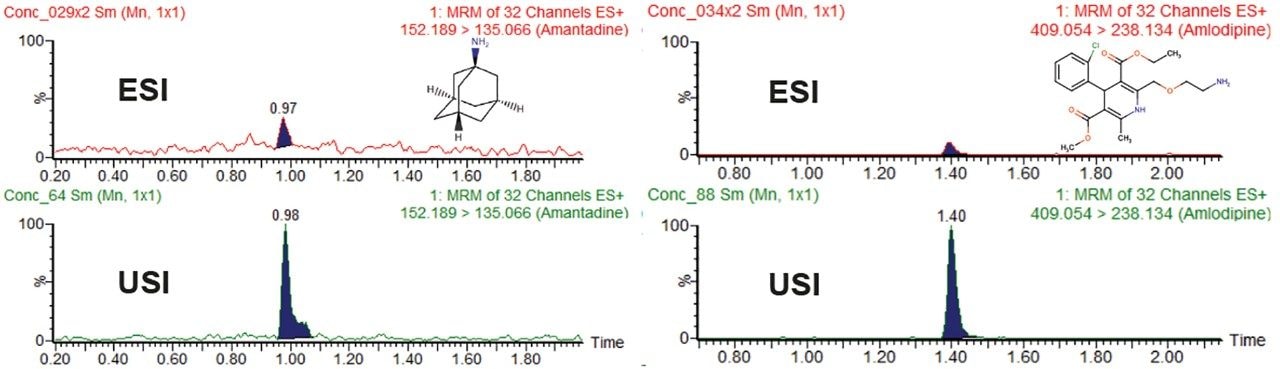
A compound library consisting of 156 compounds was automatically tuned by flow injection using the MassLynx application manager, QuanOptimize. Following MRM tuning, the compounds were screened using a short chromatographic gradient with data collected in positive as well as negative ionization modes of ESI and UniSpray. The data were processed in TargetLynx using minimal smoothing. Over 70% of the compounds had greater or comparable peak area response in USI compared to ESI (defined as the ratio of USI to ESI response above 1.0) and are summarized in Figure 2. Plots of USI/ESI response with respect to such chemical properties as pKa and LogP showed no discernible trend (Figure 3). This, with the correlation results in Figure 3C, suggests UniSpray ionization is not discriminatory and behaves similarly to ESI for the compounds in the conditions tested. To test ionization efficiency at lower concentration levels, samples were further diluted to 0.1 nM. Figure 4 shows two examples, amantadine and amlodipine, where increased ionization was observed in Unispray ionization relative to ESI. It is believed that the combination of the droplet size after impact on the pin as well as the Coănda effect and other contributions that help to enhance desolvation aid in the increased ionization and sampling efficiency of UniSpray ionization. For a more detailed discussion, please visit www.waters.com/unispraymechanisms.
To test the robustness of UniSpray ionization for matrix samples, a subset of compounds from the library were spiked into acetonitrile-precipitated human serum. Five vials, each with a different set of compounds, were injected in sequence onto the chromatographic column. Peak areas for injections 6–1800 are plotted and then summarized in Figures 5 and 6 representing nearly six days of continuous operation. The RSD for the 37 compounds tested ranged from 1.9–16. Higher RSD was observed for compounds that were at the lower end of detection.
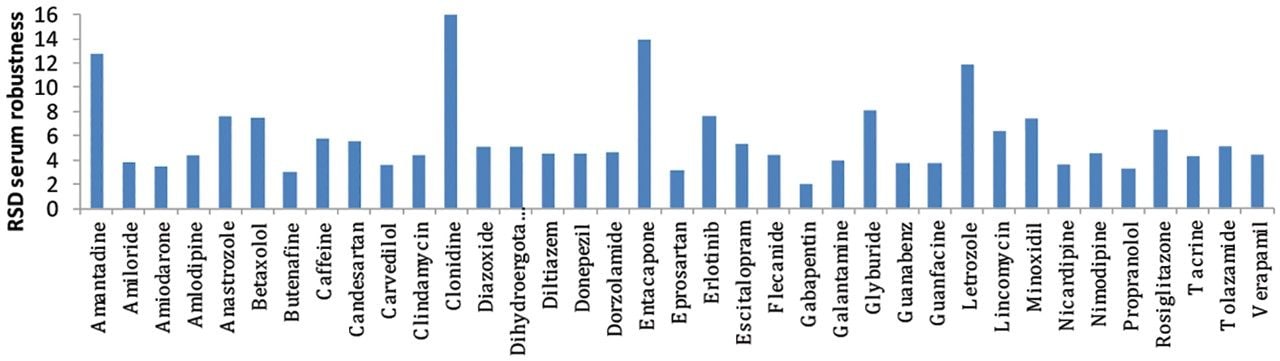
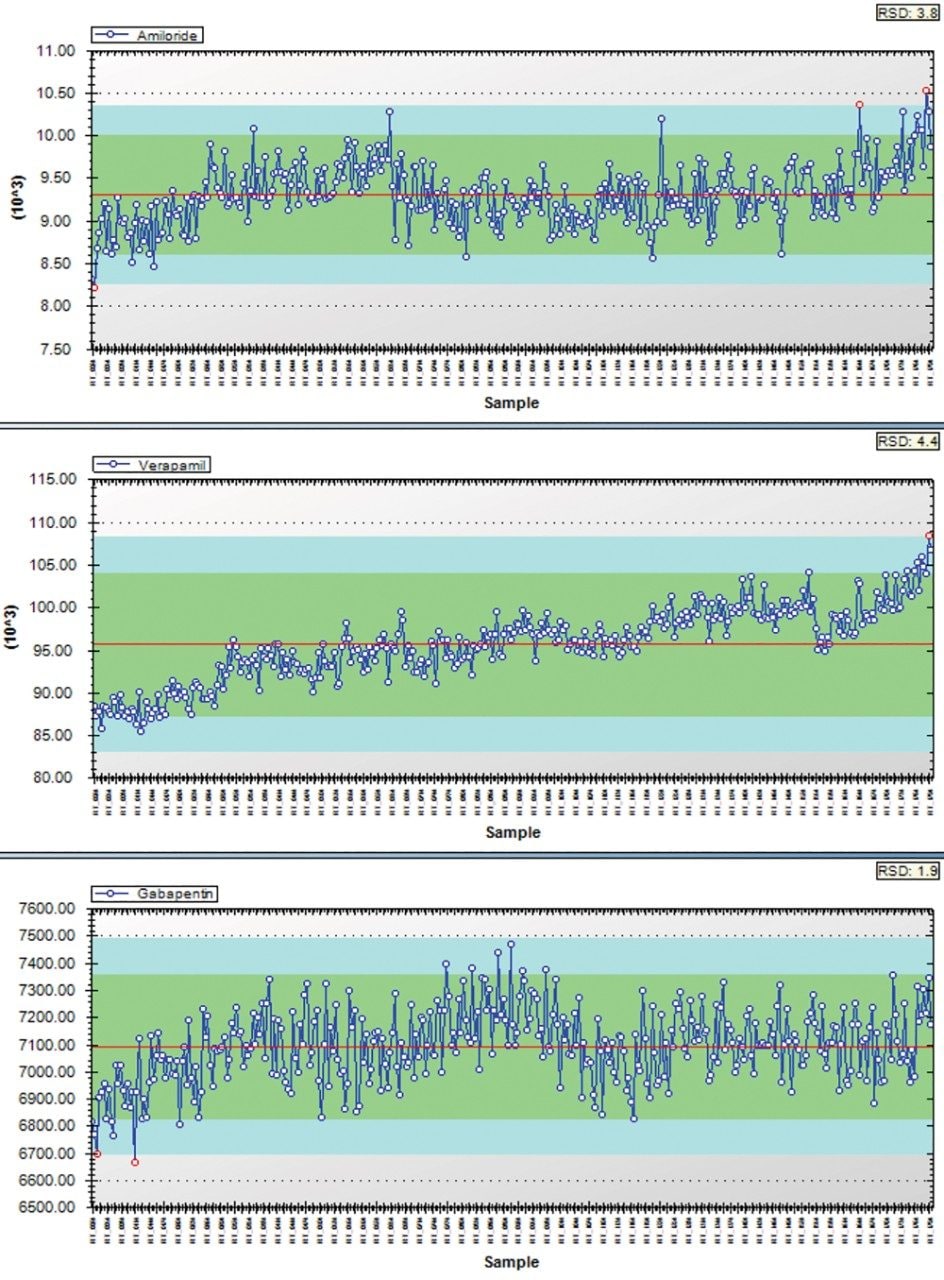
UniSpray is an ionization technique that produces results that are qualitatively similar to those of electrospray, however, increased desolvation and subsequent sampling of ions in the source enhanced the signal of the majority of the compounds in this study. The chromatographic peak area ratio of Unispray ionization to ESI ranged from 0.3 to 11.4 and was an overall average of 2.1 times greater in UniSpray for the 156 compounds tested. Finally, system robustness in human serum was tested over six days of continuous operation with peak area RSDs ranging from 1.9–16%.
720006278, May 2018