This is an Application Brief and does not contain a detailed Experimental section.
This work assess the performance of an ACQUITY UPLC H-Class PLUS System for related substances analysis of metoclopramide hydrochloride (HCl) by measuring and comparing system suitability parameters and related substances results to the data acquired on an ACQUITY UPLC H-Class System.
The ACQUITY UPLC H-Class PLUS System produced comparable system suitability and related substances results to that of the ACQUITY UPLC H-Class System. The results of the successful method performance verification demonstrate that the ACQUITY UPLC H-Class PLUS System is a reliable and robust instrument that generates analyses with excellent precision and reproducibility, ensuring that methods validated on the original ACQUITY UPLC H-Class System can be easily replicated on the ACQUITY UPLC PLUS Series Platform.
The ACQUITY UPLC H-Class PLUS System provides comparable results to the ACQUITY UPLC H-Class System with exceptional reproducibility and transferability of validated methods.
Analytical methods used for the testing of drug substances and drug products must be verified to remain in compliance with current Good Manufacturing Practices (cGMP). Method performance is verified by measuring system suitability parameters such as chromatographic resolution, peak tailing, and repeatability of the peak areas and retention times.¹ A verified analytical method is considered acceptable for quality control and will ensure that the system performs correctly at the time of use.
In this work, we assess the performance of an ACQUITY UPLC H-Class PLUS System for related substances analysis of metoclopramide hydrochloride (HCl) by measuring and comparing system suitability parameters and related substances results to the data acquired on an ACQUITY UPLC H-Class System.
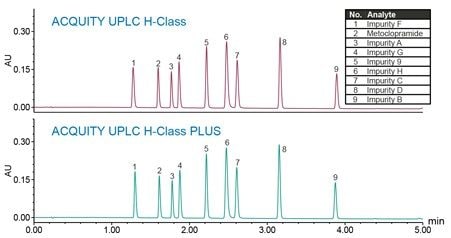
The LC method for related substances of metoclopramide HCl, previously developed in the application note 720005026EN,² was run on an ACQUITY UPLC H-Class PLUS System. Comparative studies utilizing the chromatographic data from a 0.06 mg/mL standard solution with metoclopramide API and related substances is demonstrated (Figure 1). The chromatographic separation replicated on an ACQUITY UPLC H-Class PLUS System was comparable with the original results performed on an ACQUITY UPLC H-Class System.
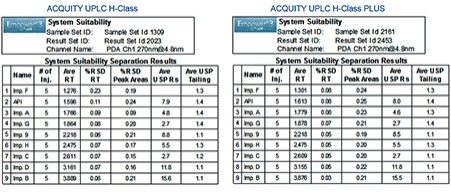
Performance of the method on the ACQUITY UPLC H-Class PLUS System was verified by evaluating system suitability of five replicate injections according to the specifications defined in the USP General Chapter <621> Chromatography³ and compared to the data acquired on the ACQUITY UPLC H-Class System. As demonstrated in Figure 2, system suitability results acquired on both systems meet the USP success criteria including repeatability of retention times, peak areas, USP peak tailing, and USP resolution.
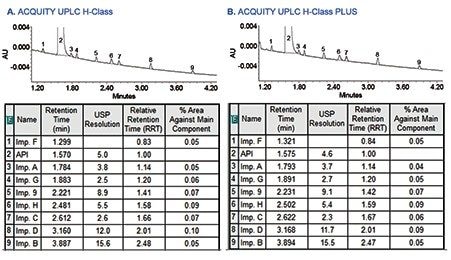
A sample solution containing metoclopramide API spiked with 0.05% of related substances was run on both the ACQUITY UPLC H-Class and ACQUITY UPLC H-Class PLUS Systems to evaluate comparability of the related substances results. As shown in Figure 3, the assay results for related substances acquired on the ACQUITY UPLC H-Class PLUS System were comparable to the original method conducted on the ACQUITY UPLC H-Class System. In addition, the relative retention time (RRT) values calculated for each related substance with respect to the API retention time were comparable.
The ACQUITY UPLC H-Class PLUS System produced comparable system suitability and related substances results to that of the ACQUITY UPLC H-Class System. The results of the successful method performance verification demonstrate that the ACQUITY UPLC H-Class PLUS System is a reliable and robust instrument that generates analyses with excellent precision and reproducibility, ensuring that methods validated on the original ACQUITY UPLC H-Class System can be easily replicated on the ACQUITY UPLC PLUS Series Platform.
Maziarz, M.; McCarthy, S.M.; Wrona, M. Improving Effectiveness of Method Development for Metoclopramide HCl and Related Substances Using a Systematic Screening Protocol. Waters Application Note, 720005026, April 2014.
USP General Chapter <621> Chromatography, USP, United States Pharmacopeia Convention, Official.
720006230, April 2018