This application note presents the transfer of an HPLC method for related substances of metoclopramide HCl from different LC systems to an ACQUITY Arc System.
Analytical methods used for testing of pharmaceutical raw materials and finished products are often transferred across organizations or to contract partners that utilize instruments from different vendors. It is therefore essential for the analytical laboratories to successfully transfer these methods between different instruments to ensure product consistency and compliance with the regulations. Effective method transfer generates equivalent results for the same analysis independent of the instrument, laboratory, or the resources. This is important to eliminate the need to revalidate the method, which is time-consuming and costly.
Transfer of chromatographic methods between different LC systems, especially from different manufacturers, can be a challenging task. Often, these instruments have different system volumes, which may cause poor chromatographic separation and peak distortion in gradient methods. Therefore, system volume differences must be accounted for when transferring gradient methods between different LC systems to achieve the same separation.
In this work, we present the transfer of an HPLC method for related substances of metoclopramide HCl from different LC systems to an ACQUITY Arc System. Additionally, we will demonstrate method improvement by scaling this method to columns with a smaller particle size. The success of the method transfer will be measured by examining chromatographic data and the system suitability results against USP specifications.2,3 Overall, we will show that the ACQUITY Arc System, enabled by its Arc Multi-flow path technology, can efficiently replicate an established method from any LC platform without altering the method’s gradient table. The ACQUITY QDa Detector coupled to an ACQUITY Arc System provides quick peak identification of sample components by mass detection.
Solutions preparation
The related compounds of metoclopramide HCl used in this study are listed in Table 1. Separate stock solutions were prepared in methanol at 1.0 mg/mL. A metoclopramide stock solution was diluted with water to 0.5 mg/mL and spiked with related substances at 1.0% level.
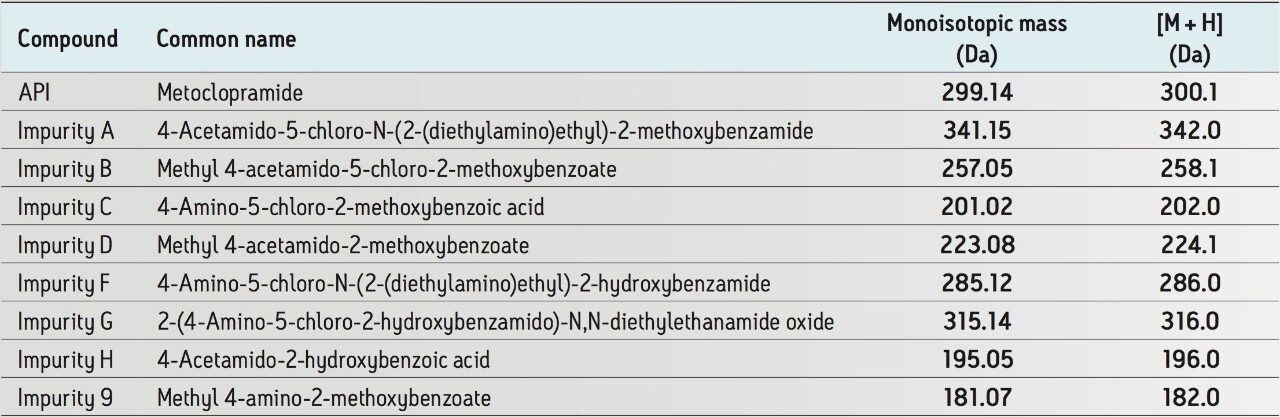
For a system suitability standard, each stock solution was transferred to one vial and diluted with standard diluent (50:50 methanol/water) to 0.05 mg/mL of each analyte. The mixture was then diluted with standard diluent (50:50 methanol/water) to 15 μg/mL for system suitability determination.
Method conditions
LC systems for method transfer:
The HPLC method for related substances of metoclopramide HCl was scaled to 3.5 and 2.5 μm particle size columns and then to UPLC using the Waters ACQUITY UPLC Columns Calculator.1 The flow rate, injection volume, and gradient times were geometrically scaled to preserve separation integrity.
|
Column: |
XSelect CSH C18 4.6 x 150 mm, 5 μm |
|
Flow rate: |
2.9 mL/min |
|
Injection volume: |
10.0 μL |
|
Separation: |
Gradient |
|
Step |
Time(minutes) |
Solvent A(%) |
Solvent B(%) |
|---|---|---|---|
|
1 |
Initial |
95.0 |
5.0 |
|
2 |
15.00 |
40.0 |
60.0 |
|
3 |
16.50 |
40.0 |
60.0 |
|
4 |
16.80 |
95.0 |
5.0 |
|
5 |
21.00 |
95.0 |
5.0 |
|
Column: |
XSelect CSH C18 3.0 x 100 mm, 3.5 μm |
|
Flow rate: |
1.2 mL/min |
|
Injection volume: |
2.8 μL |
|
Separation: |
Gradient |
|
Step |
Time(minutes) |
Solvent A(%) |
Solvent B(%) |
|---|---|---|---|
|
1 |
Initial |
95.0 |
5.0 |
|
2 |
10.00 |
40.0 |
60.0 |
|
3 |
11.00 |
40.0 |
60.0 |
|
4 |
11.20 |
95.0 |
5.0 |
|
5 |
14.00 |
95.0 |
5.0 |
|
Column: |
XSelect CSH C18 3.0 x 75 mm, 2.5 μm |
|
Flow rate: |
1.2 mL/min |
|
Injection volume: |
2.1 μL |
|
Separation: |
Gradient |
|
Step |
Time(minutes) |
Solvent A(%) |
Solvent B(%) |
|---|---|---|---|
|
1 |
Initial |
95.0 |
5.0 |
|
2 |
7.50 |
40.0 |
60.0 |
|
3 |
8.25 |
40.0 |
60.0 |
|
4 |
8.40 |
95.0 |
5.0 |
|
5 |
10.50 |
95.0 |
5.0 |
|
Solvent A: |
0.1% formic acid in water |
|
Solvent B: |
0.1% formic acid in methanol |
|
Purge/Sample wash: |
50:50 water/methanol |
|
Seal wash: |
90:10 water/acetonitrile |
|
UV detector: |
λ range: 210–400 nm, derived at 270 nm, sampling rate: 20 pts/sec |
|
Mass detector: |
ACQUITY QDa Detector (Alliance, ACQUITY Arc, ACQUITY UPLC H-Class) |
|
Ionization mode: |
ESI+, ESIAcquisition |
|
range: |
100–440 m/z |
|
Sampling rate: |
10 pts/sec |
|
Capillary voltage: |
Pos: 0.8 kV, Neg: 0.8 kV |
|
Cone voltage: |
15 V |
|
Probe temperature: |
600 °C |
|
Data: |
Centroid |
|
System Control, Data Acquisition, and Analysis: |
Empower 3 FR2 CDS Software |
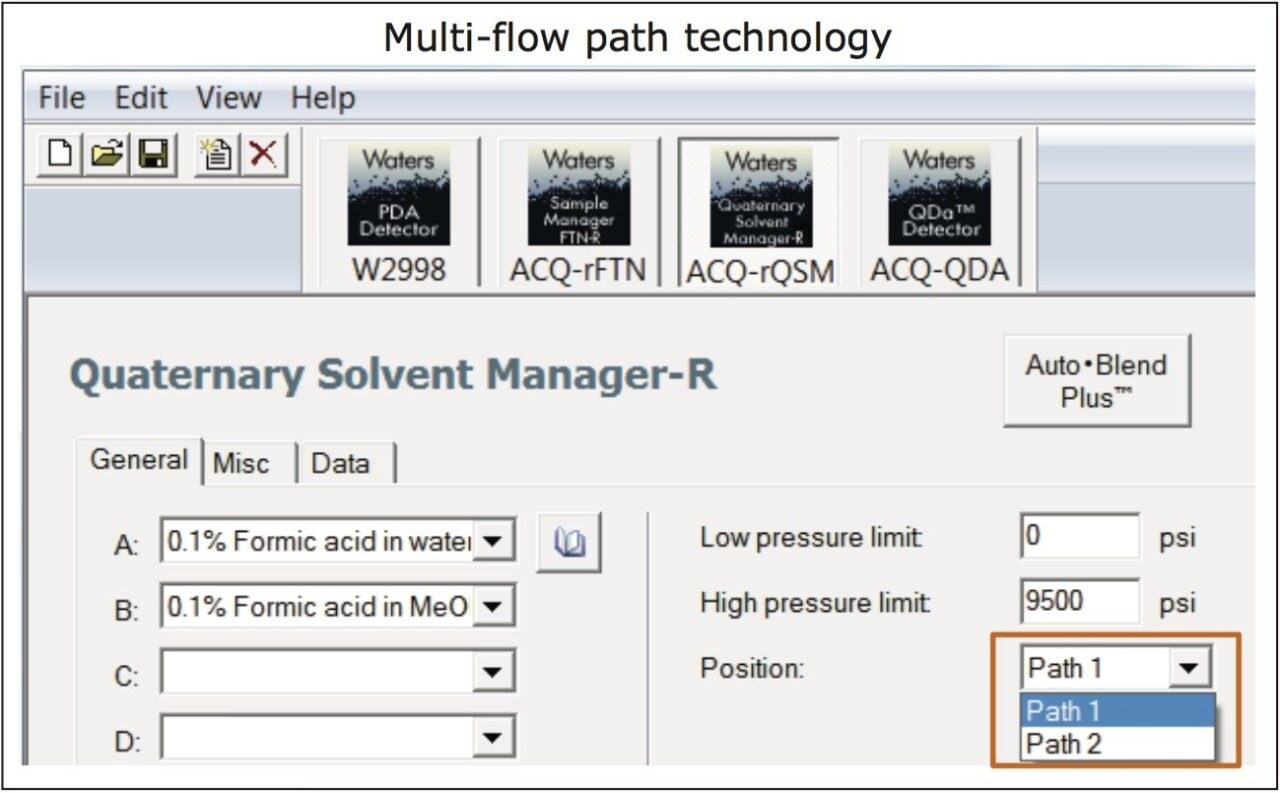
For successful transfer of the gradient methods between different LC systems, the dwell volume of each system should be characterized and compensated for in efforts to maximize the success of the technology transfer. The ACQUITY Arc System is enabled by Arc Multi-flow path technology (Figure 1), which allows the user to select the fluidic path to emulate dwell volume and mixing behavior of different LC systems. By selecting path 1 or path 2, established LC methods can be easily replicated without any manual intervention or the need to alter the gradient table.
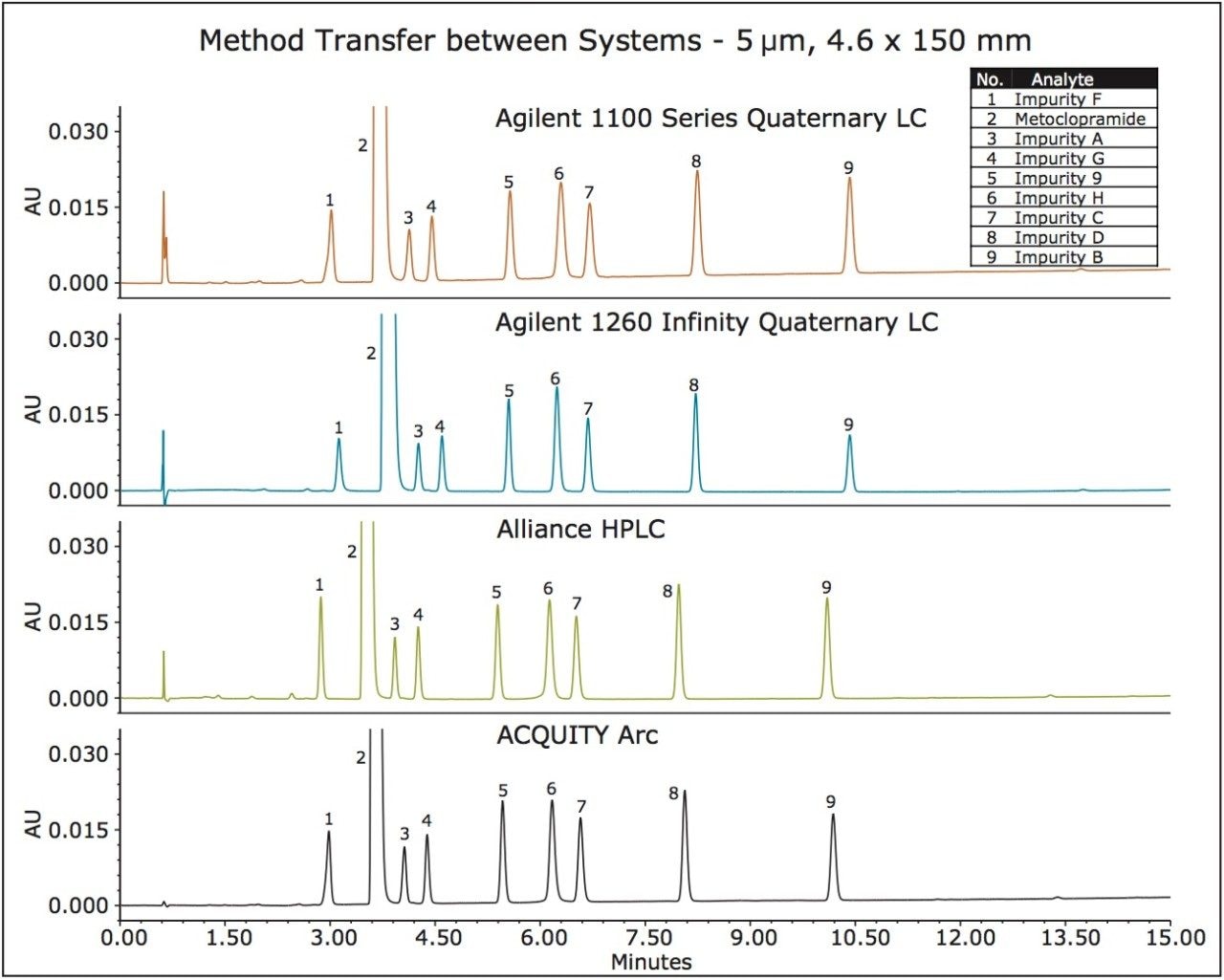
In this study, the HPLC assay method for related substances of metoclopramide HCl was run on an Agilent 1100, Agilent 1260, Alliance HPLC Systems, and an ACQUITY Arc System using fluidic path 1. The chromatographic data of a sample containing metoclopramide API with 1.0% of related substances acquired on all four systems is shown in Figure 2. The chromatographic separation produced on an ACQUITY Arc System is comparable with the results obtained on the Agilent and Alliance HPLC Systems.
Confirming peak identity
The mass spectra data acquired using an ACQUITY QDa Detector coupled with an ACQUITY Arc System was used to confirm the identity of metoclopramide and related substances by mass detection (Figure 3).
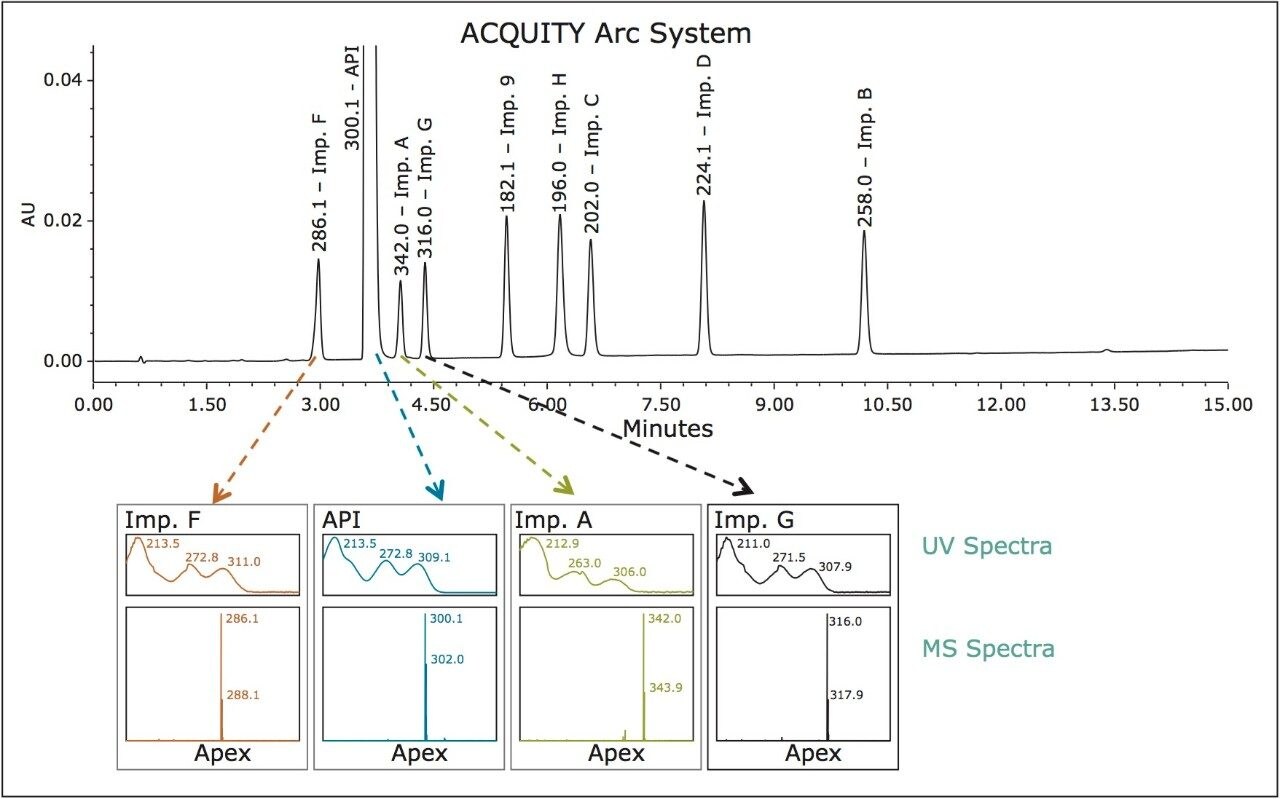
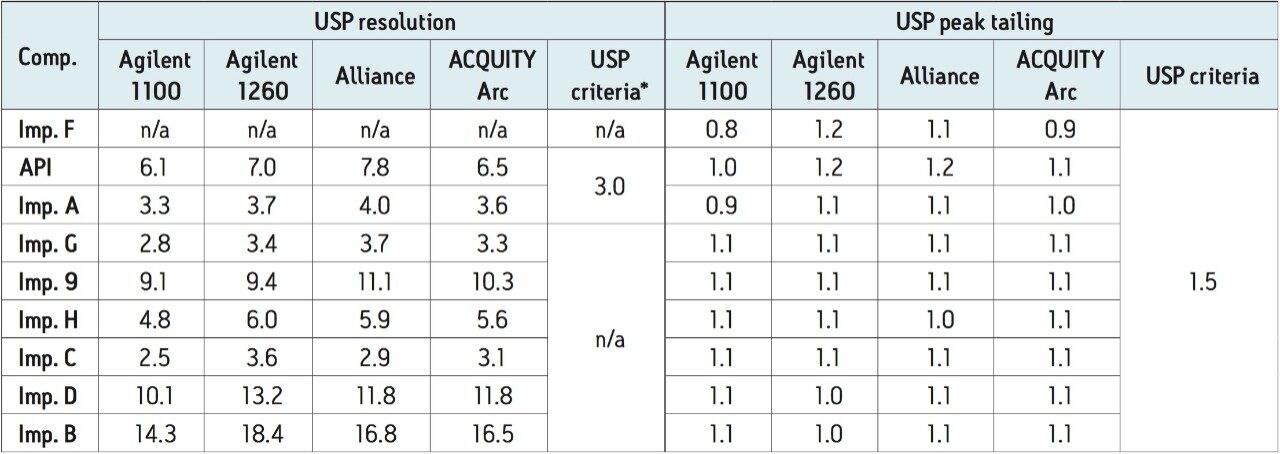
Performance of the HPLC method run on the ACQUITY Arc System was verified by evaluating system suitability results of five replicate injections of the system suitability standard against the requirements listed in the USP Monograph for Metoclopramide Hydrochloride2 and USP General Chapter, <621> Chromatography.3 The system suitability results were also compared to the results obtained on the Agilent 1100, Agilent 1260, and Alliance HPLC Systems.
The recently updated USP Monograph for Metoclopramide Hydrochloride lists a method for analysis of several organic impurities (A, B and D).2 The monograph states that the USP resolution between metoclopramide API and Imp. A must not be less than 3.0. The USP resolution of our method run on all four systems met the USP criteria (Table 2). Additionally, a USP resolution of ≥ 3.1 was observed for all peaks on the ACQUITY Arc System. The USP tailing factors were less than 1.5 and comparable across the systems (Table 2). The retention times and peak areas repeatability of the method run on the ACQUITY Arc System were substantially lower than the specifications defined in the General Chapter, <621> Chromatography of less than 2.0% RSD and comparable with the results acquired on other LC systems (Table 3).

Method transfer to the columns with smaller particles results in shorter runs times and faster linear velocities that can be used to increase throughput and productivity. In order to properly transfer a method to a column with smaller particle size, the resolving power of the original method must be maintained. This is accomplished by using a column that has the same ratio of column length (L) to particle size (dp) as the original method. The flow rate, injection volume, and gradient times are scaled properly to maintain the same separation integrity and can be achieved using the Waters ACQUITY UPLC Columns Calculator.
In this study, the HPLC method for related substances of metoclopramide HCl was scaled to the 3.5 and 2.5 μm particle columns and run on the ACQUITY Arc System. Scaling the HPLC method to columns with smaller particles has resulted in a reduced run time and solvent consumption, while maintaining integrity of the chromatographic separation (Figure 4). Migration to a 3.5 μm particle size column reduced analysis time by 33% and solvent consumption by 72%. Scaling to a 2.5 μm particle size column reduced analysis time and solvent consumption by 50% and 79%, respectively.
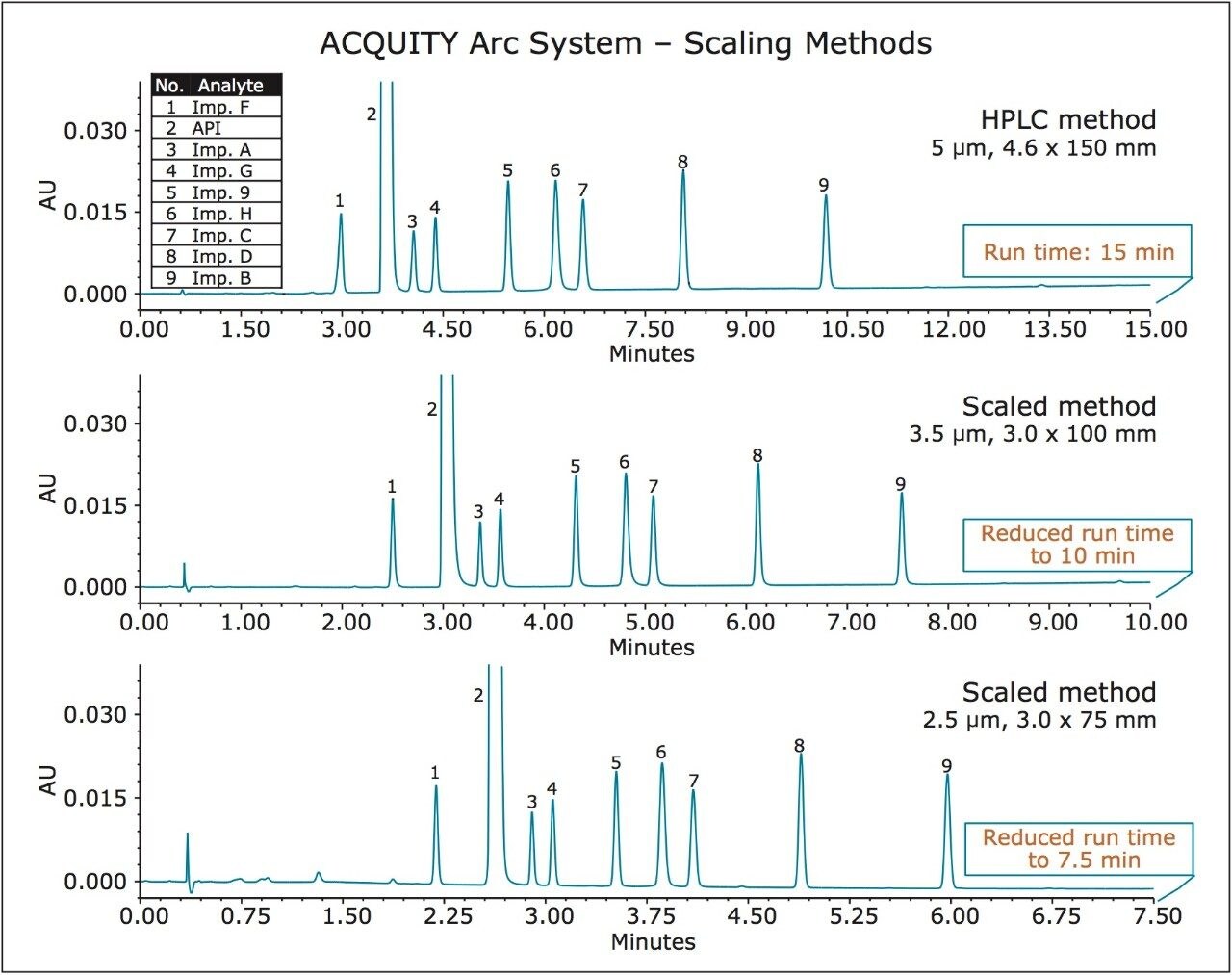
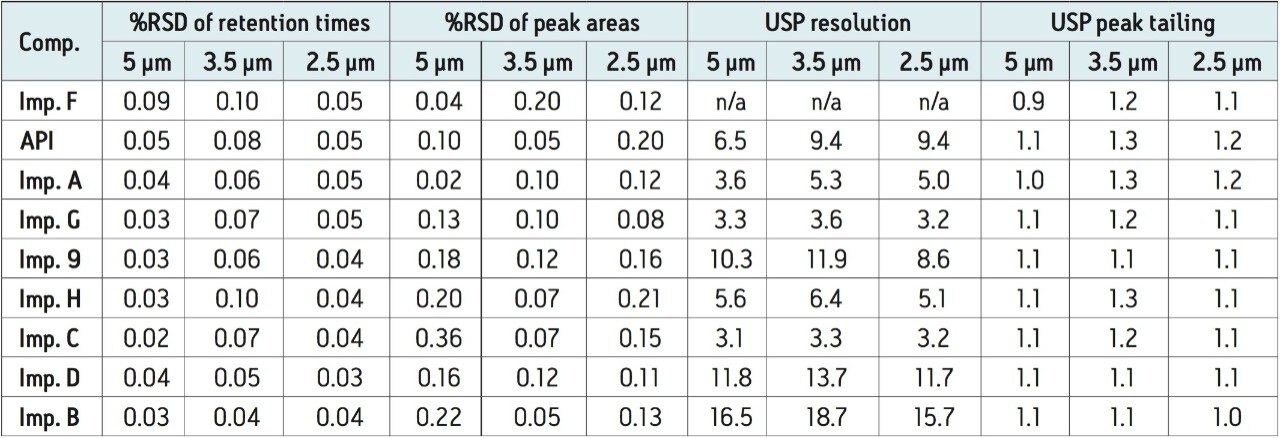
Comparison of the system suitability results of five replicate injections of the system suitability standard for the HPLC and scaled methods run on an ACQUITY Arc System are shown in Table 4. The retention times and peak areas repeatability of the scaled methods were substantially lower than the USP specifications of less than 2.0% RSD and comparable to the HPLC method. The USP resolution values and the USP peak tailing factors were also comparable.
Overall, the data shows that the ACQUITY Arc System easy accepts and improves methods to increase efficiency and productivity. It accommodates columns with 2.5 to 5.0 μm particles. This provides the flexibility to support a wide-range of LC applications.
The ACQUITY Arc System successfully replicated the assay method for related substances of metoclopramide HCl run on an Agilent 1100 Series LC, Agilent 1260 Infinity Quaternary LC, and Alliance HPLC Systems. The ACQUITY QDa Detector coupled to an ACQUITY Arc System enabled quick peak identification using mass spectral data. Migration from 5.0 to 3.5 and 2.5 μm particle size columns reduced analysis time and solvent consumption. The ACQUITY QDa Detector coupled to an ACQUITY Arc System enabled quick peak identification using mass spectral data.
Enabled by a unique Multi-flow path technology, the ACQUITY Arc System easily accepts and replicates methods from a variety of platforms without compromising method integrity. The ACQUITY Arc System provides powerful LC performance and secures the lab’s investment by ensuring integration with new technologies such as the ACQUITY QDa Detector and Empower Chromatography Data System (CDS) software. Overall, the ACQUITY Arc System allows efficient method transfer from any LC platform, as well as flexible method optimization.
720005558, January 2016