
This is an Application Brief and does not contain a detailed Experimental section.
This technology brief demonstrates how the solid-core UHPLC particle chemistry CORTECS Phenyl Column can be used to test neonicotinoid insecticides by UPLC-MS/MS, using either an acetonitrile or methanol gradient separation method.
CORTECS Phenyl Columns highlight neonicotinoid separation benefits using acetonitrile and methanol gradients.
Neonicotinoid insecticides are a class of neuro-active insecticides developed in the late 1980s and marketed beginning in the 1990s, which now represent 24% of the global market for insecticides. One of the neonicotinoids, imidacloprid, is the most widely used insecticide in the world. In the U.S., neonicotinoids are used on nearly all of the corn and canola crops, on the majority of cotton, sorghum, and sugar beet crops, and on fruits and vegetables including apples, oranges, leafy greens, tomatoes, potatoes, rice, and nuts.
The neonicotinoids are effective in controlling harmful and destructive crop pests, but recent studies have raised concerns about the neonicotinoids harming bees and other pollinators. In April, 2015, the U.S. Environmental Protection Agency (EPA) temporarily halted new uses for four of the neonicotinoids (imidacloprid, dinotefuran, clothianidin, and thimethoxam) until new bee data is submitted and pollinator risk assessments are complete. The EPA’s preliminary risk assessment for imidacloprid identified an imidacloprid residue level of 25 ppb, above which effects on pollinator hives are likely to be seen.
Phenyl columns were chosen for neonicotinoid analysis because they are the column of choice for the separation of structurally similar compounds with aromatic ring structures, such as the neonicotinoids. Columns packed with solid-core particles and MS/MS detection were chosen because low detection limits are required in order to study the low ppb levels of neonicotinoid residues in samples such as plants, pollens, and soil. The solid-core particles provide increased efficiency when compared to fully, porous particles, giving narrower and taller peaks, thus decreasing the limit of detection.
A mixture of seven neonicotinoids were separated on a CORTECS Phenyl Column, 2.7 μm, 2.1 x 50 mm (p/n 186008319) using an ACQUITY UPLC H-Class System and Xevo TQD. Two different gradient methods were used: 5% to 70% acetonitrile in water with 0.1% formic acid, and 5% to 70% methanol in water with 0.1% formic acid, both at a flow of 0.5 mL/min.
Using either acetonitrile or methanol as the organic solvent in the gradient gave baseline resolution between all seven neonicotinoid peaks as shown in Figure 1. Although baseline resolution is not required when using MS/MS detection, it does eliminate the possibility of matrix effects between potentially co-eluting analytes.
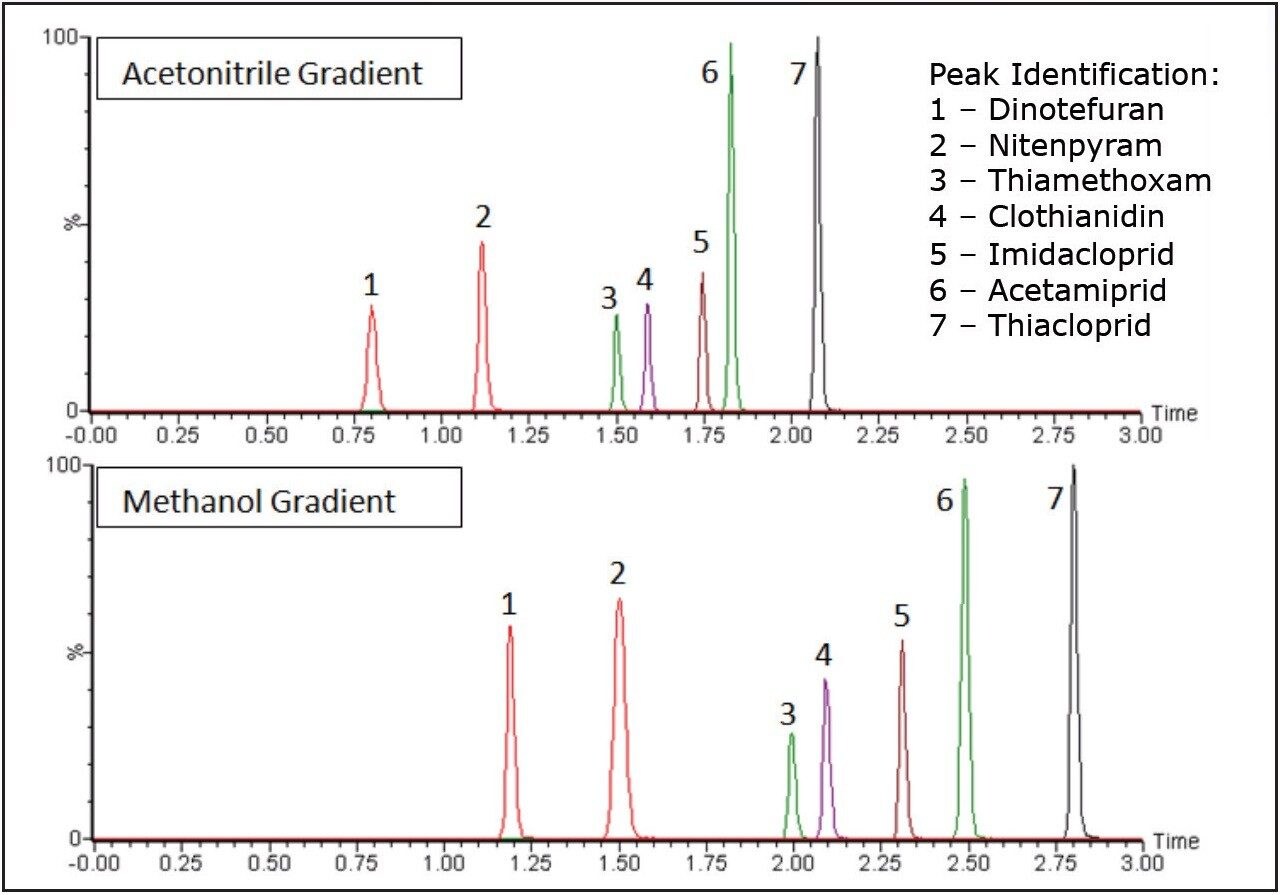
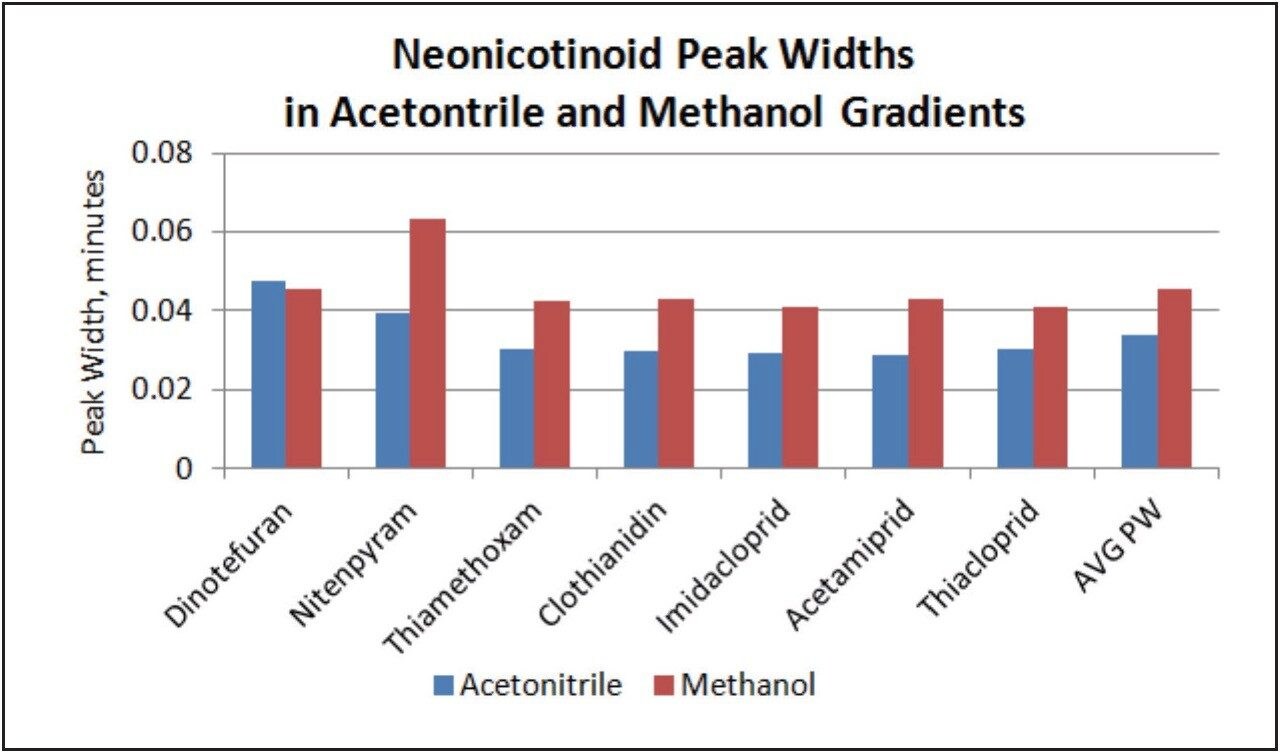
Peak widths were smaller when using the acetonitrile gradient for all but one neonicotinoid, as shown in Figure 2. This would lead to lower detection limits for the neonicotinoids when using acetonitrile in the mobile phase. The smaller peak widths seen when using acetonitrile also give a greater peak capacity and better resolution values.
Using methanol does have one potential advantage. More retention is seen, i.e., the retention factor (k’) values are larger, when using methanol. See Figure 3. This increase in retention when using methanol can be explained by the acetonitrile mobile phase suppressing the pi-pi interactions between the phenyl ligand and the aromatic neonicotinoid analytes, when compared to a methanol mobile phase. This increase in retention may be advantageous when trying to separate the neonicotinoids from the sample matrix.
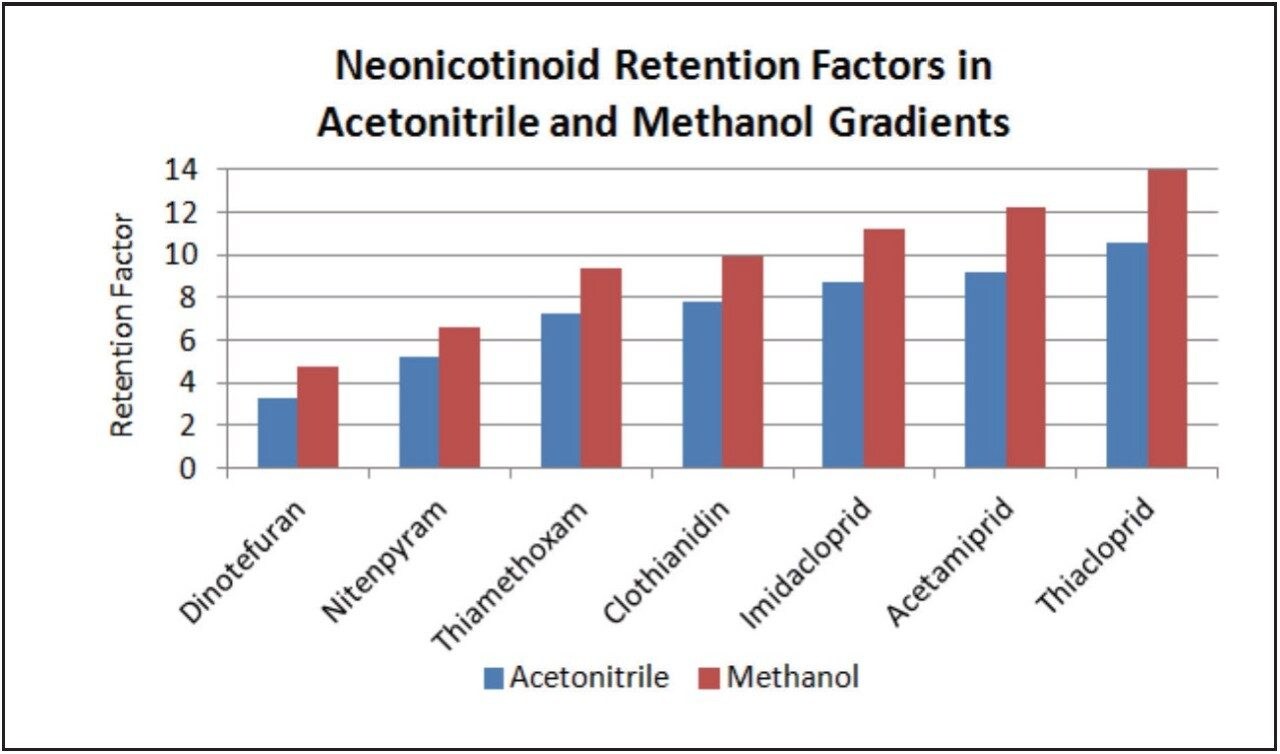
Complete separation of all seven neonicotinoids is seen on the CORTECS Phenyl Column. To maximize detection limits, peak capacity, and resolution, an acetonitrile gradient is recommended. To maximize retention factors, a methanol gradient is recommended.
720005624, Aprill 2016