
The gradient delay or dwell volume of a system can affect the separation and must, therefore, be considered when transferring a method across instruments from different vendors. With the ACQUITY UPLC H-Class System, Gradient SmartStart can be used to adjust the gradient delay to match other instrumentation. This feature, included in the instrument method, was successfully used to replicate a method for the analysis of clozapine and related impurities on an Agilent 1100 Series LC System. The separation on the ACQUITY UPLC H-Class System matched the system suitability criteria observed on the HPLC instrumentation, including relative retention time and USP resolution. In addition, correlation was also observed for the % of each impurity and the total % of all impurities in the sample. This example illustrates the ability to successfully transfer an HPLC method to an ACQUITY UPLC H-Class System and maintain all acceptance criteria without the need to make any adjustments to the method.
Transferring existing analytical LC methods between instruments from different manufacturers is often challenging. The main components of an instrument (the pump, the injector, the column compartment) can have different design characteristics, which might affect the fidelity of the separation, particularly in gradient separations. For example, chromatographic instruments may use singleor dual-piston pumps; low- or high-pressure mixing. These pumping characteristics affect the volume delay or the time the gradient reaches the head of the column.
In order to adjust for differences in solvent delivery between instruments, the dwell volume of a chromatographic system is typically measured when transferring gradient separations.1,2 The dwell volume can then be used for adjustment of the gradient.3 Adjustments are often performed manually, requiring changes to the gradient table, however dwell volume compensation can also be accomplished through software automation. For example, the ACQUITY UPLC H-Class System utilizes a feature that controls the gradient start (at injection, pre injection, or post-injection) without changing the contents of the gradient table. This software feature, Gradient SmartStart, enables the analyst to easily factor in dwell volume differences between systems, thereby increasing the likelihood of success in analytical gradient methods transfer.
Clozapine Resolution Mixture was purchased from United States Pharmacopeia. Five milligrams of the sample was dissolved in 12.5 mL of a 80:20 (v/v) methanol/water solution. The sample was vortexed to ensure complete dissolution. The final concentration of the sample was of 400 μg/mL.
|
LC conditions |
|
|
LC Systems (Table 1): |
Agilent 1100 Series LC System with Agilent 1100 DAD Detector Agilent 1290 Infinity LC System with Agilent 1290 DAD Detector ACQUITY UPLC H-Class System with CH-A and ACQUITY UPLC PDA Detector |
|
Sample: |
Clozapine USP System Resolution Standard (USP catalog number 1142108) |
|
Column: |
ZORBAX Eclipse XDB C18, 3.5 μm, 4.6 x 150 mm |
|
Column Temperature: |
30 °C (with mobile phase pre-heating) |
|
Mobile phase A: |
0.1% Formic acid in water |
|
Mobile phase B: |
0.1% Formic acid in acetonitrile |
|
Flow rate: |
1.5 mL/min |
|
Injection volume: |
5 μL |
|
Wavelength: |
254 nm |
|
Weak needle wash: |
90:10 Water/acetonitrile |
|
Strong needle wash: |
10:90 Water/acetonitrile |
|
Seal wash: |
50:50 Water/methanol |
|
Gradient: |
5-30% B in 5 min, 30-95% B in 4 min |
|
Data management: |
|
|
Chromatography software: |
Empower 3 FR2 |
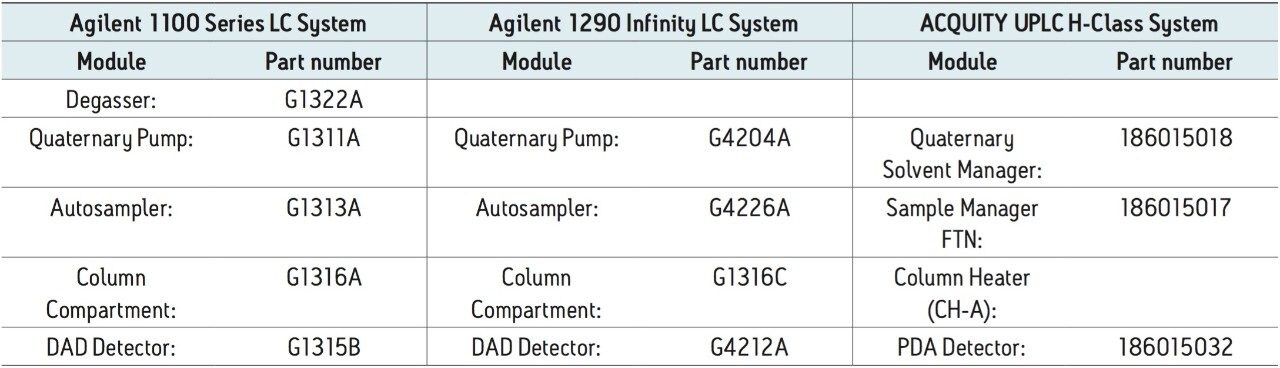
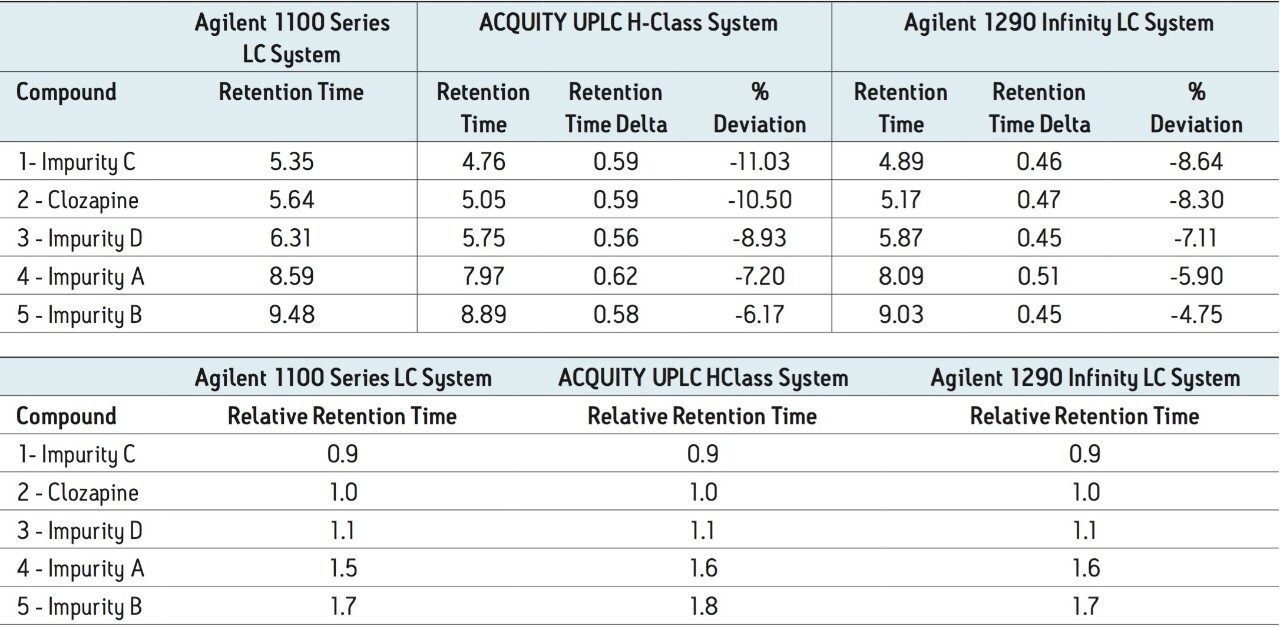
The analysis of clozapine and related compounds was performed on an Agilent 1100 LC Series System (Figure 1). The method was transferred to an ACQUITY UPLC H-Class System and an Agilent 1290 Infinity LC Quaternary System. On all systems, the averages of five replicate injections were used for analysis. The separation on both the ACQUITY UPLC H-Class and the Agilent 1290 Infinity LC systems produced shorter retention times than observed on the Agilent 1100 LC Series System. The retention time shifts (Table 2) ranged from 0.56-0.62 minutes on the ACQUITY UPLC H-Class System and 0.45-0.51 minutes on the Agilent 1290 Infinity Quaternary LC System. Both sets of values represent differences of 4-9%, which are outside the desired variation of less than 3%.4
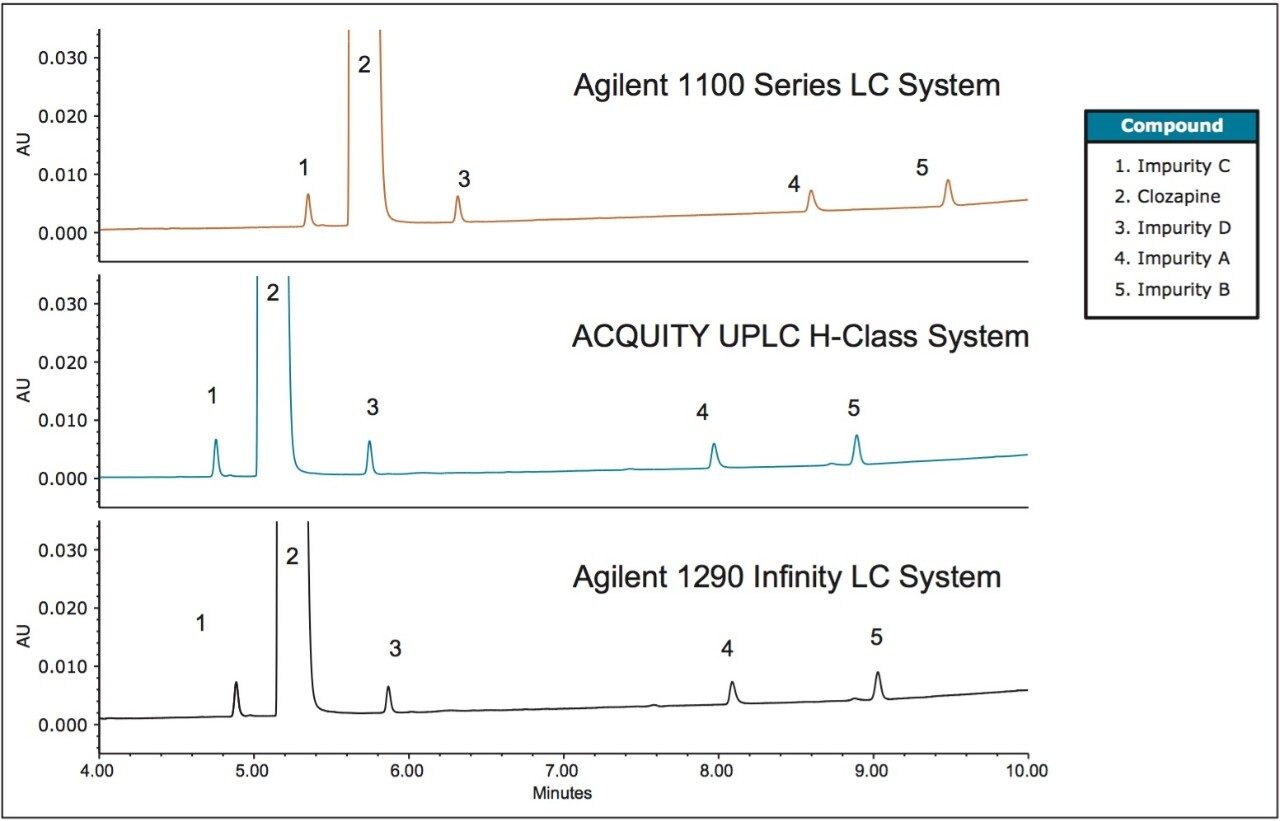
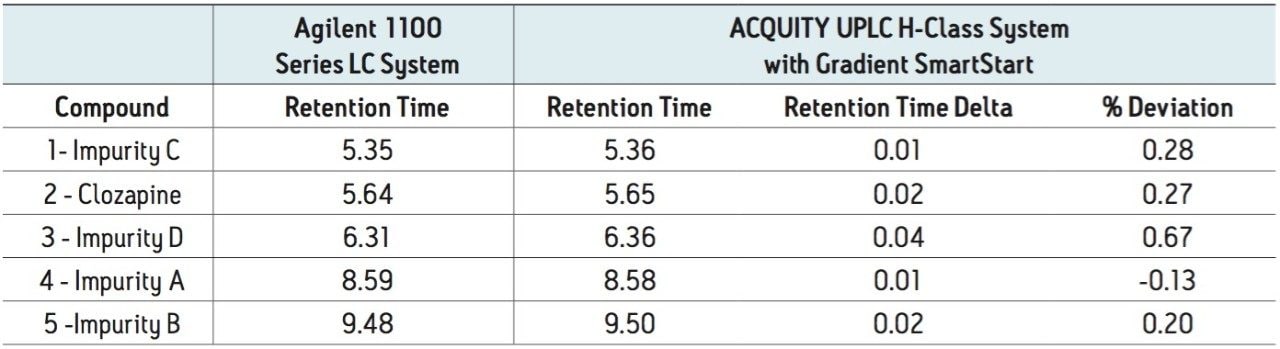
Although retention times are commonly used to characterize an analytes’ response, relative retention times (RRT) are often used for system suitability requirements to address variability between LC systems, as is the case in the USP monograph for clozapine.5 Therefore, the RRT of the impurities were calculated for the previously described analyses. The RRT for the earlier eluting peaks were the same across all systems. However, differences were observed for the RRT of the later eluting peaks on both the ACQUITY UPLC H-Class and the Agilent 1290 Infinity LC systems, as compared to the Agilent 1100 Series LC System. For this analysis, the same RRTs were required the criteria,5 therefore the difference was outside the acceptable window (Table 2b).
Retention time variability for gradient separations can, in part, be attributed to the dwell volume differences of the instruments. The dwell volume, or the volume of the system between the point at which the solvents are mixed to the inlet of the column,6 can affect the gradient delay and the separation. For this reason, the dwell volume of each system was measured by placing a union and appropriate restrictor in place of the column.
A mobile phase containing a UV absorber (propyl paraben in acetonitrile) was run from 0–100 % in 10 minutes. The dwell volume was calculated from this measurement (Figure 2). For the systems used in this study, the dwell volumes were found to be approximately 1.290 mL for the Agilent 1 100 LC System and 0.375 mL for the ACQUITY UPLC H-Class System.
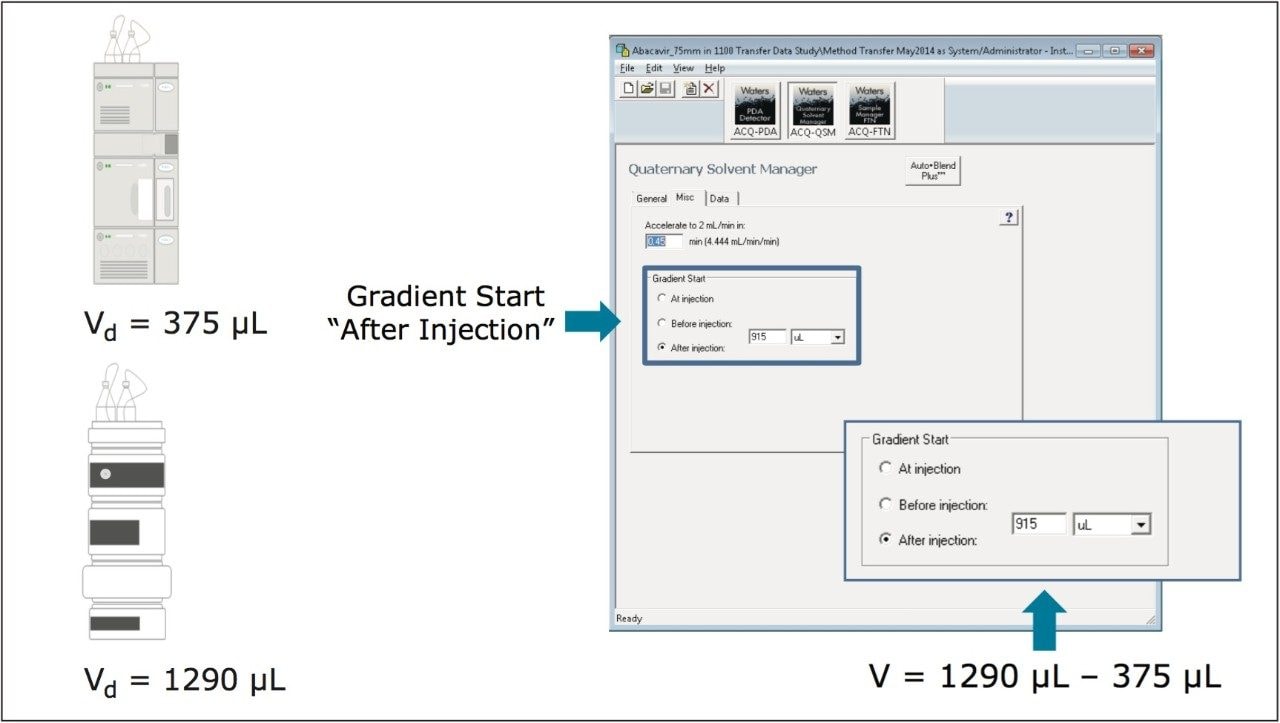
Based on the measurements previously described, the method was re-run on the ACQUITY UPLC H-Class System with a post injection delay of 915 μL (the difference between the measured dwell volumes). The post injection delay was entered directly in units of volume using Gradient SmartStart feature in the instrument method (Figure 2). The results (Figure 3) produced chromatography that met both retention time and RRT criteria: the difference in retention time decreased approximately 10x from 0.5–0.6 minutes to less than 0.05 minutes (Table 3a and b), which correlated to a difference of less than 1%. In addition, the relative retention times matched those obtained on the Agilent 1100 Series LC System.
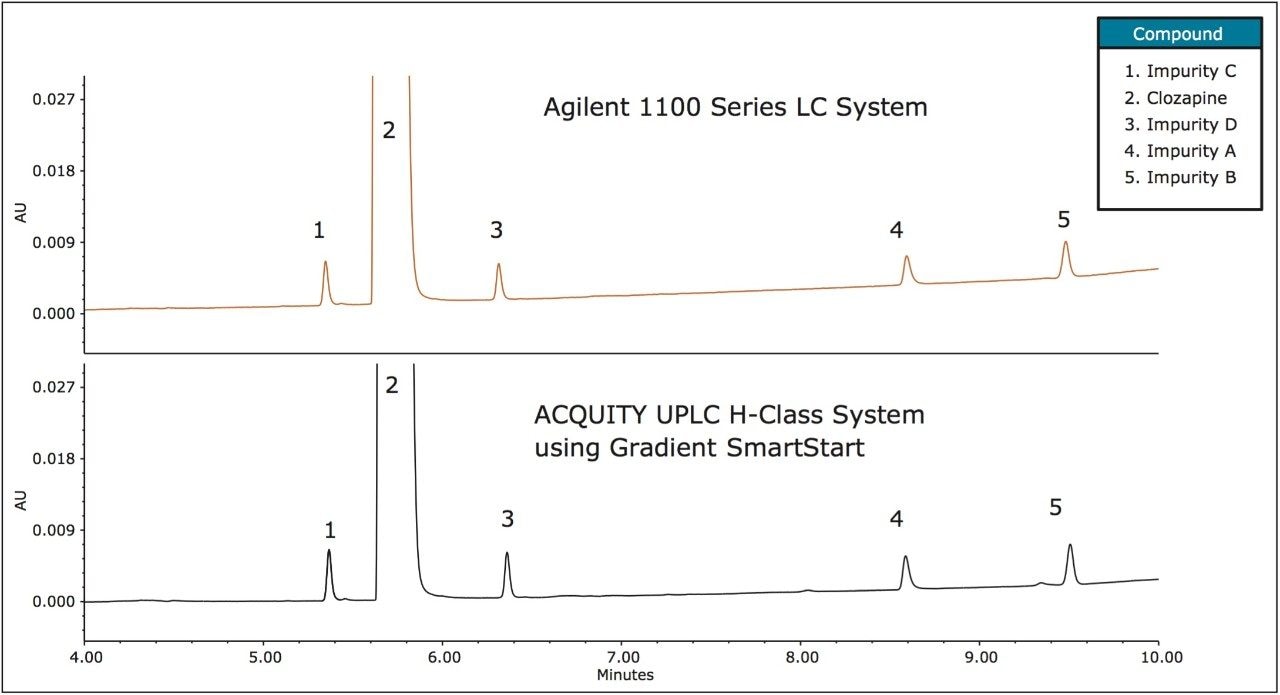

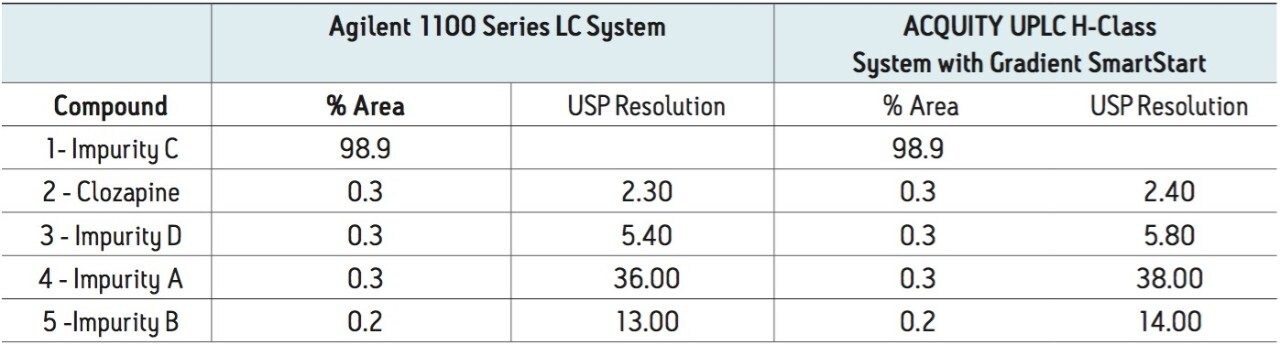
While the testing on the ACQUITY UPLC H-Class System using Gradient SmartStart met the relative retention time criteria, there are a number of additional values that are of importance in API testing (Table 4). System suitability requirements often include resolution, particularly for critical pairs. In this example, due to the lower dispersion of the ACQUITY UPLC H-Class System, the USP resolution improved for all known compounds as compared to the Agilent 1100 Series LC System, with improvements between 4–8%. Additional acceptance criteria for organic impurity testing is often the % of each impurity and the total % of all impurities in the sample.5 For the study conducted, no change in the % API or the % of each impurity was observed in the two analyses, indicating comparable relative quantitative values.
The gradient delay or dwell volume of a system can affect the separation and must, therefore, be considered when transferring a method across instruments from different vendors. With the ACQUITY UPLC H-Class System, Gradient SmartStart can be used to adjust the gradient delay to match other instrumentation. This feature, included in the instrument method, was successfully used to replicate a method for the analysis of clozapine and related impurities on an Agilent 1100 Series LC System. The separation on the ACQUITY UPLC H-Class System matched the system suitability criteria observed on the HPLC instrumentation, including relative retention time and USP resolution. In addition, correlation was also observed for the % of each impurity and the total % of all impurities in the sample. This example illustrates the ability to successfully transfer an HPLC method to an ACQUITY UPLC H-Class System and maintain all acceptance criteria without the need to make any adjustments to the method.
720005332, June 2015