The ACQUITY Arc System is an LC platform designed to bridge the gap between HPLC and UPLC, allowing users to seamlessly transfer methods across laboratories. The introduction of the ACQUITY Arc System recognizes the need to emulate legacy HPLC techniques, but also offers the advantage of adopting UHPLC technology if desired. An SEC method was successfully transferred from an Agilent 1100 Series HPLC to the ACQUITY Arc System without changing any method parameters. The ACQUITY Arc System demonstrated a high degree of reproducibility, which is important when verifying product consistency. Finally, the transition from SEC-HPLC to SEC-UHPLC showed improved resolution and a shorter run time while maintaining comparable peak area percentages to those obtained under HPLC conditions.
Size exclusion chromatography (SEC) is a common technique used in the pharmaceutical industry for the analysis of biotherapeutics, including monoclonal antibodies. SEC is often used throughout the lifecycle of a drug product, from discovery through commercialization. Because analytical methods are commonly transferred to various laboratories within an organization, or to contract organizations throughout a product’s lifetime, regulatory guidelines require that method equivalency be demonstrated between laboratories to ensure product quality and consistency. As with any assay used for release testing, it is important that the instrumentation used for analysis be robust and easy to deploy across laboratories. The ACQUITY Arc System is an LC platform designed to bridge the gap between HPLC and UPLC, allowing users to seamlessly transfer methods across laboratories. Legacy HPLC methods can be easily replicated and UHPLC methods can be readily adopted with the use of Arc Multi-flow path technology.1 This study uses a monoclonal antibody to assess SEC method transfer from an Agilent 1100 Series instrument to the ACQUITY Arc System. Peak area and retention time will be used as metrics to demonstrate equivalency between platforms, after which system repeatability of the ACQUITY Arc System will be evaluated. Finally, the HPLC method used to demonstrate method transfer will be updated to a UHPLC method to yield better resolution and a faster run time.
|
LC systems: |
ACQUITY Arc System with 2489 UV/Vis Detector, flow path 1 Agilent 1100 Series LC System with quaternary pump and DAD detector |
|
Absorption wavelength: |
280 nm |
|
Sampling rate: |
20 Hz |
|
Column temp.: |
30 °C |
|
Mobile phase: |
0.02 M sodium phosphate, 0.3 M sodium chloride, pH 6.8 |
|
Sample temp.: |
5 °C |
|
Injection volume: |
30 μL |
|
HPLC column: |
Tosoh TSK gel G3000 SWXL 250 Å, 5 μm, 7.8 mm x 300 mm |
|
Flow rate: |
0.5 mL/min |
|
Method length: |
35 min |
|
UHPLC column: |
XBridge Protein BEH SEC, 200A, 3.5 μm, 7.8 mm x 300 mm (p/n 176003596) |
|
Flow rate: |
0.714 mL/min |
|
Method length: |
24.5 min |
Empower 3 CDS Software, SR2
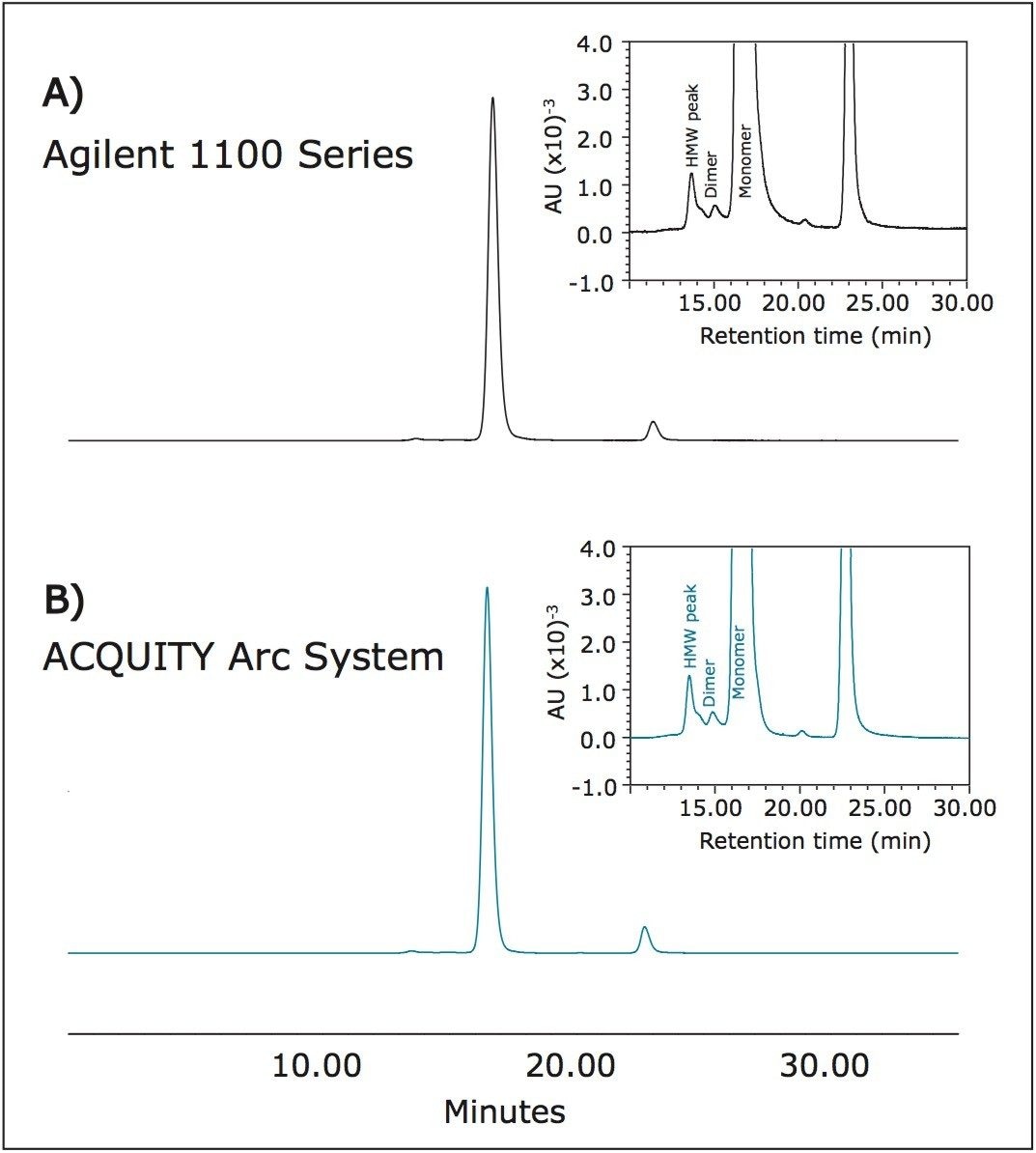
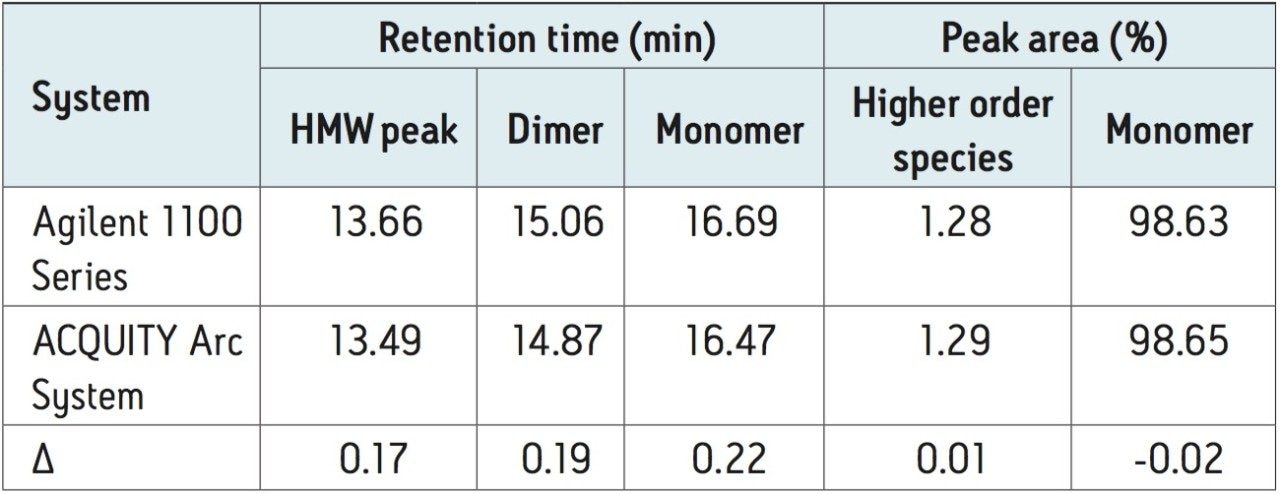
A monoclonal antibody, rituximab, was prepared at 1 mg/mL in mobile phase and used to study HPLC method transfer from an Agilent 1100 Series instrument to the ACQUITY Arc System. The SEC method used was taken from the USP Medicines Compendium,2 and although the compendium is now discontinued, the method is representative of a typical SEC analysis that would be used in the industry. To establish a benchmark chromatogram, rituximab was separated using a Tosoh SEC column on an Agilent 1100 Series instrument (Figure 1A). This same method was transferred to the ACQUITY Arc System and run using fluidic Path 1 under identical method conditions (Figure 1B). Visual inspection of the chromatograms shows a high degree of similarity. Evaluation of retention time and peak area percent, as reported in Table 1, confirms this agreement. The shift in retention time between instruments was approximately 0.2 minutes, but more importantly, peak area percent remained unchanged.
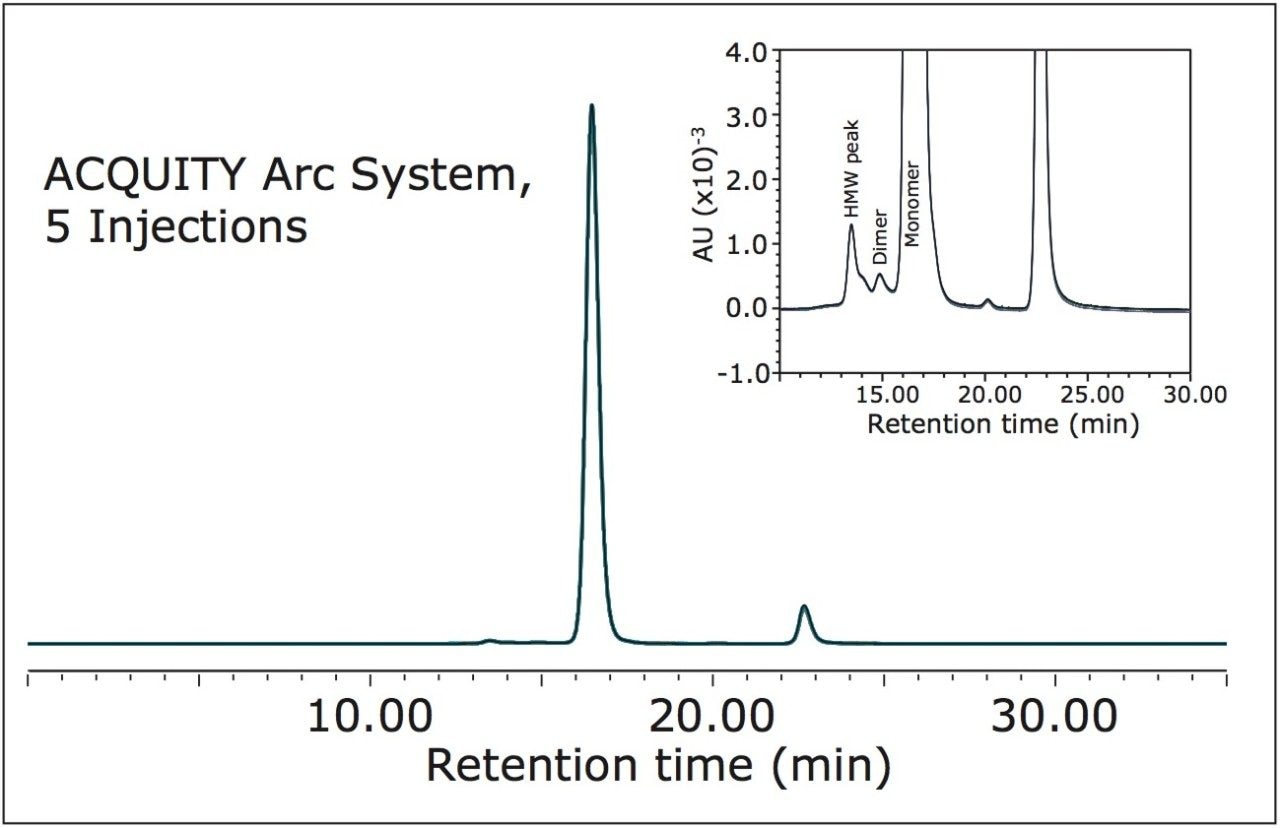
With industry standards demanding a high degree of product consistency and the need to meet suitability requirements, demonstrating instrument reproducibility is important. As shown in Figure 2, overlays of five injections on the ACQUITY Arc System are nearly indistinguishable when using the method parameters described above. Table 2 provides quantitative analysis of this data by reporting retention time, peak area percent, and resolution results for both systems.

The ACQUITY Arc System enables users to operate under both HPLC and UHPLC conditions on a single platform. To take advantage of this, the Tosoh SEC column was replaced with a XBridge Protein BEH SEC Column. Decreasing particle size requires that additional method parameters be scaled appropriately as well. Because column dimensions are the same, the new flow rate, F2, is proportional to the ratio of particle diameter according to the following equation:
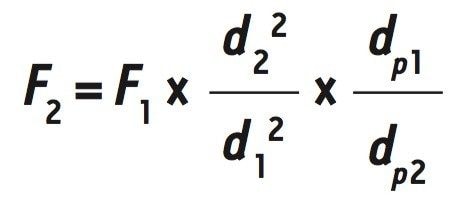
Where F1 is the old flow rate, d1 and d2 are the old and new internal column diameters, and dp1 and dp2 are the old and new particle sizes. By using the equation above, a new flow rate of 0.714 mL/min was calculated. The adjusted flow rate can be used to determine the new run time, t2 , according to the following equation:
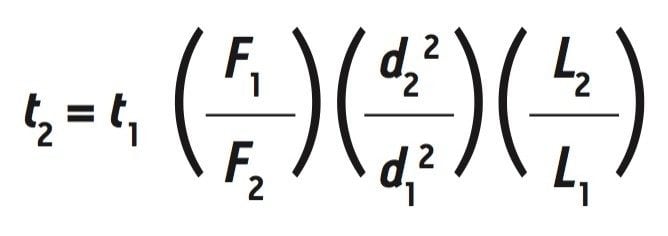
Where t1 is the old run time and L1 and L2 are the old and new column lengths. The adjusted run time was calculated to be 24.5 min. These new method conditions meet the guidelines for the USP’s allowable adjustments,3 which allows for the SEC method to be updated without requiring re-validation.
Rituximab was separated under UHPLC conditions and results were compared to those reported by HPLC. Improved resolution between the dimer and main peak are seen when comparing the HPLC data (Figure 3A) to the UHPLC data (Figure 3B). UHPLC conditions also led to sharper peaks with earlier elution times. Although UHPLC conditions showed improved resolution, relative peak area percentages of the higher order species and the monomer peak remained unchanged between the two methods. This indicates that there was minimal interaction between rituximab and the stationary phase, which is true of an ideal SEC separation. Table 2 contains data comparing UHPLC results to earlier reported HPLC results from both systems.
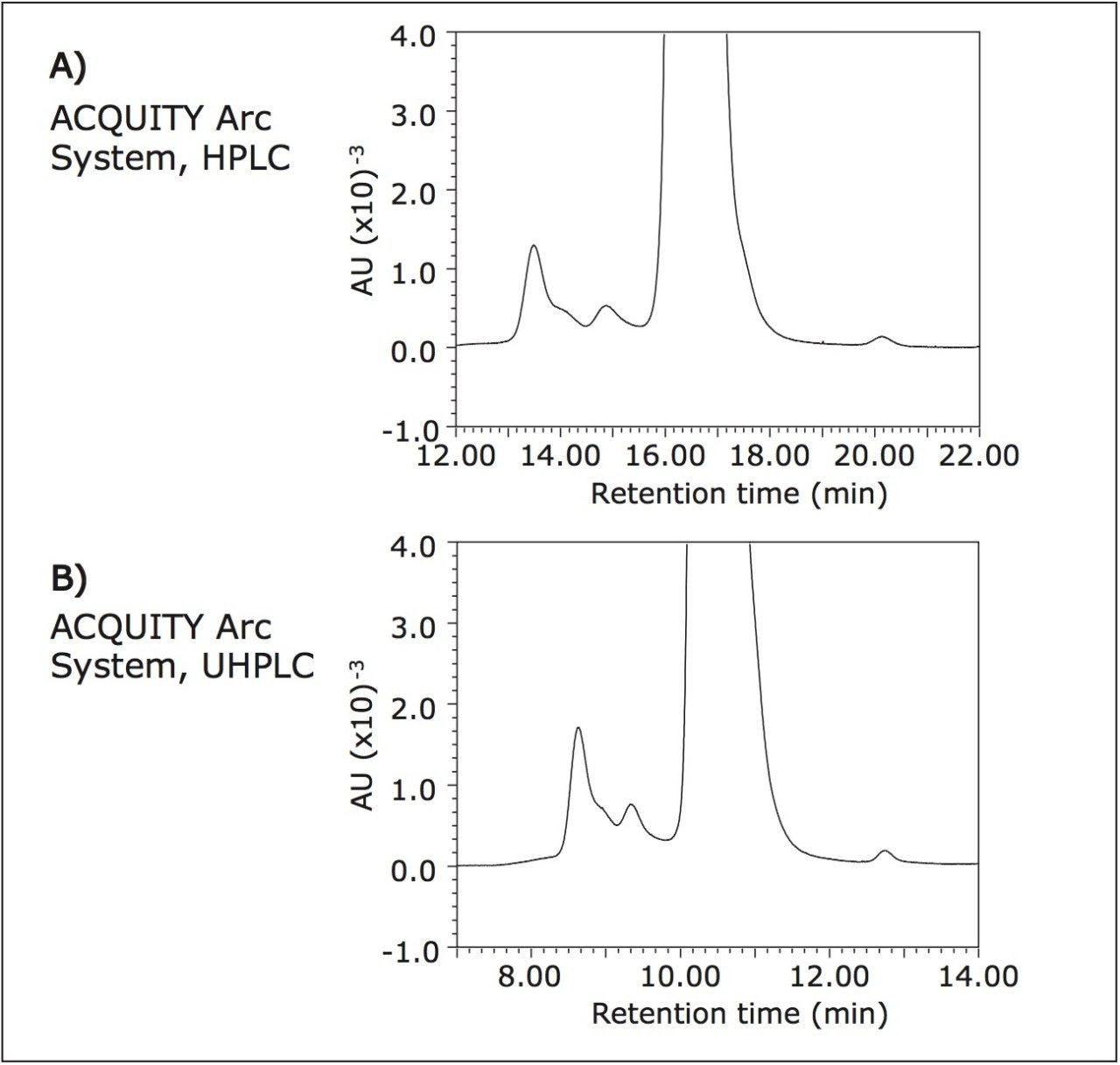
The introduction of the ACQUITY Arc System recognizes the need to emulate legacy HPLC techniques, but also offers the advantage of adopting UHPLC technology if desired. An SEC method was successfully transferred from an Agilent 1100 Series HPLC to the ACQUITY Arc System without changing any method parameters. The ACQUITY Arc System demonstrated a high degree of reproducibility, which is important when verifying product consistency. Finally, the transition from SEC-HPLC to SEC-UHPLC showed improved resolution and a shorter run time while maintaining comparable peak area percentages to those obtained under HPLC conditions.
720005510, September 2015