
This is an Application Brief and does not contain a detailed Experimental section.
This application brief demonstrates the precise and reliable analysis of standard and formulated samples of ziprasidone using the USP compendial method and hybrid column technology on an Alliance HPLC System.
Analysis of ziprasidone capsules using an Alliance HPLC System provided consistent and reproducible results.
USP compendial methods are routinely used as a basis for analyzing generic drugs. Column designations for USP methods vary depending on the monograph. Here, we demonstrate the analysis of the USP compendial method for ziprasidone HCl, which designates an L7 column type, on an Alliance HPLC System equipped with a 2998 Photodiode Array (PDA) Detector. The XBridge C8 hybrid particle was chosen to provide a more robust stationary phase for analysis over a wide range of pH and buffered mobile phase conditions, compared to silica-based particle phases. The Alliance HPLC System was used for its robust, reliable, and precise performance along with simplicity in operation, all of which are critical attributes for a system used in routine analytical laboratories.
Ziprasidone, an anti-psychotic drug, was analyzed in both standard and capsule forms on an Alliance HPLC System using the XBridge C8 L7 column. The suitability criteria described in the ziprasidone HCl USP monograph was used to monitor assay performance and evaluate a successful analysis.
The USP monograph for ziprasidone HCl designates the use of an L7 column and suggests a Zorbax RX-C8 column. An equivalent column, XBridge C8, was chosen using the Waters Column Selectivity Chart. The XBridge column employs hybrid column technology that promotes column robustness under wide pH and buffered mobile phase conditions. The USP compendial method for ziprasidone HCl was run as written using the L7 designated XBridge C8 column on an Alliance HPLC System equipped with a 2998 PDA Detector. Excellent peak shape was obtained for both the standard and formulated ziprasidone samples, as shown in Figure 1. The simplified fluidic pathway of the Alliance HPLC System enables lower system dispersion resulting in sharper peaks and high quality data.
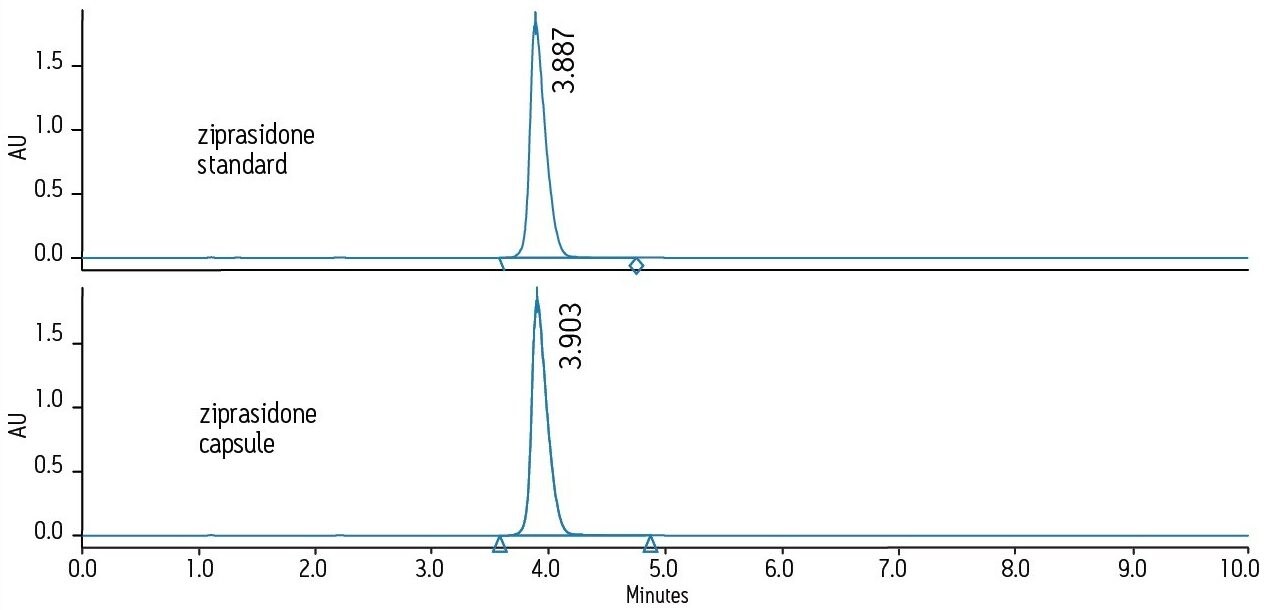
Five replicate injections of ziprasidone standard and ziprasidone capsule samples were analyzed on the Alliance HPLC System. Assay suitability criteria, including USP tailing and injection repeatability, were found to be well within specifications for both samples, demonstrating superior system performance using the compendial method, as shown in Table 1. Integrated fluidics for solvent and sample management, as well as independently driven pistons and automated solvent compressibility featured on the Alliance HPLC System provide smooth solvent flow and exceptionally consistent and reproducible results. This is demonstrated by the exceptionally low retention time and peak area %RSDs for replicate injections of ziprasidone standard and capsule samples.
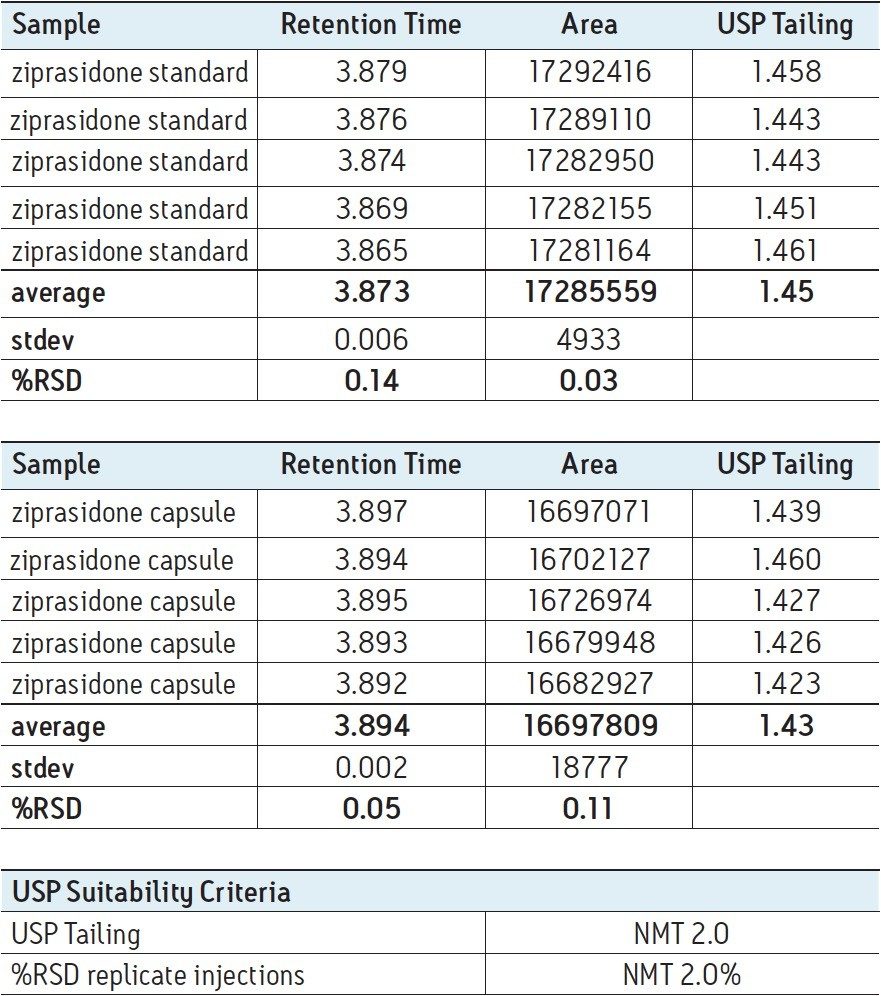
Ziprasidone standard and capsules were analyzed using the USP compendial method on an Alliance HPLC System. Using a combination of XBridge hybrid column technology with the high performance Alliance HPLC System ensures chromatographic performance well within specified suitability criteria. The use of the precise and reliable Alliance HPLC System for routine analysis of compendial methods provides high quality, reproducible results while minimizing costly instrument downtime and maintenance.
720004555, January 2013