
In this application note we have shown the analysis of chiral pesticides using ACQUITY UPC2. ACQUITY UPC2 allows high efficiency separations that can significantly increase the sample throughput when compared with traditional normal-phase separations.
The development of analytical methods for the separation of chiral compounds is important in many areas of research because it is well known that enantiomers can react differently in a chiral environment. Biochemical reactions can be stereo or enantioselective – and while one enantiomer may deliver the desired effect (referred to as the eutomer) to the target species, the other enantiomer may be less effective to the target or completely ineffective. It is estimated that 30% of pesticides on the market today have optical isomers and there are reports that 40% of the pesticides used in China are chiral.1,2 The study of enantioselectivity is important to the agricultural chemicals manufacturing industry since the knowledge of the efficacy of each individual enantiomer could facilitate a significant reduction in the total amount of pesticide applied. In order to improve our knowledge of enantioselectivity, analytical methods that provide reliable and reproducible separations in a rapid time frame are needed. Supercritical fluid chromatography (SFC) has become known as an effective chiral separations technique possessing many advantages over conventional high performance liquid chromatography (HPLC).3,4 The properties of a supercritical fluid allow high efficiency separations with shorter analysis times to be achieved. The structural complexity of new pesticides is increasing, which means that there is a greater likelihood that multiple chiral centers may be present in a molecule5,6,7 and high efficiency techniques are needed to perform successful separations.
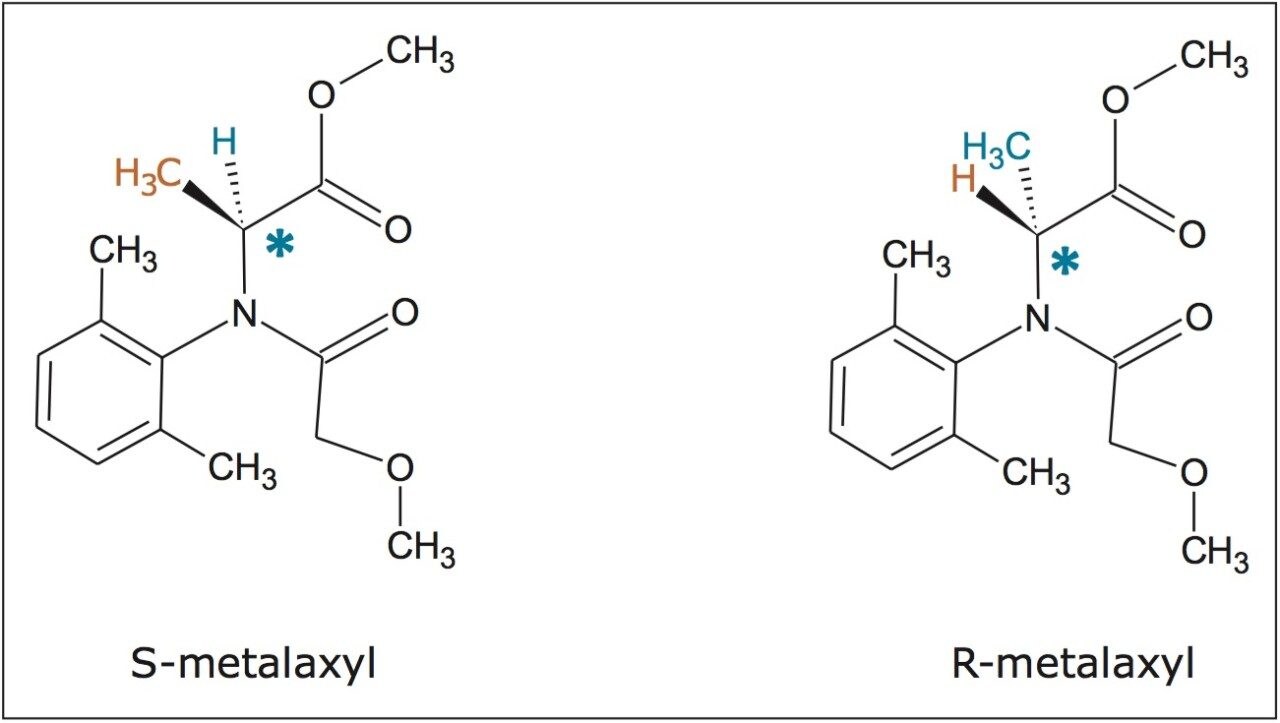
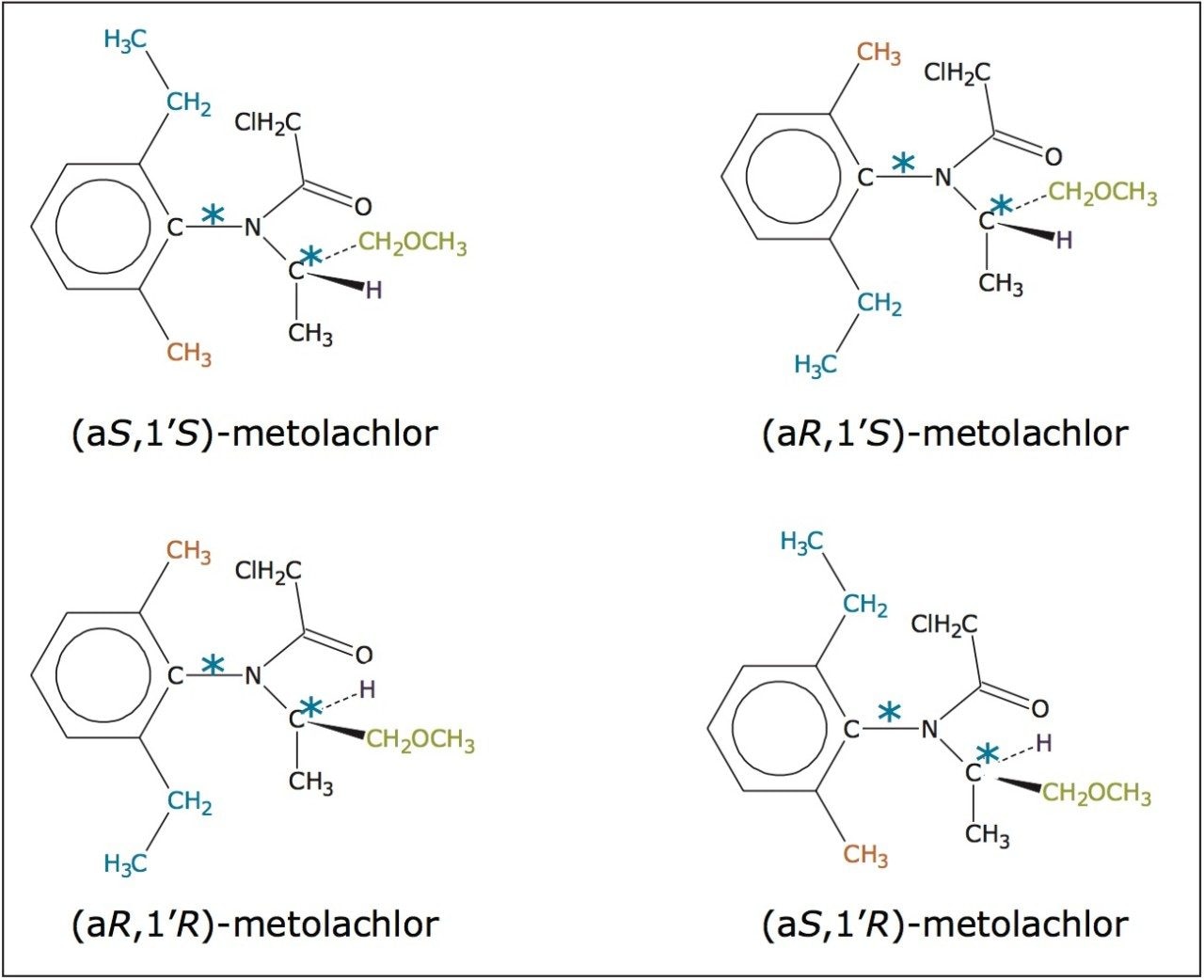
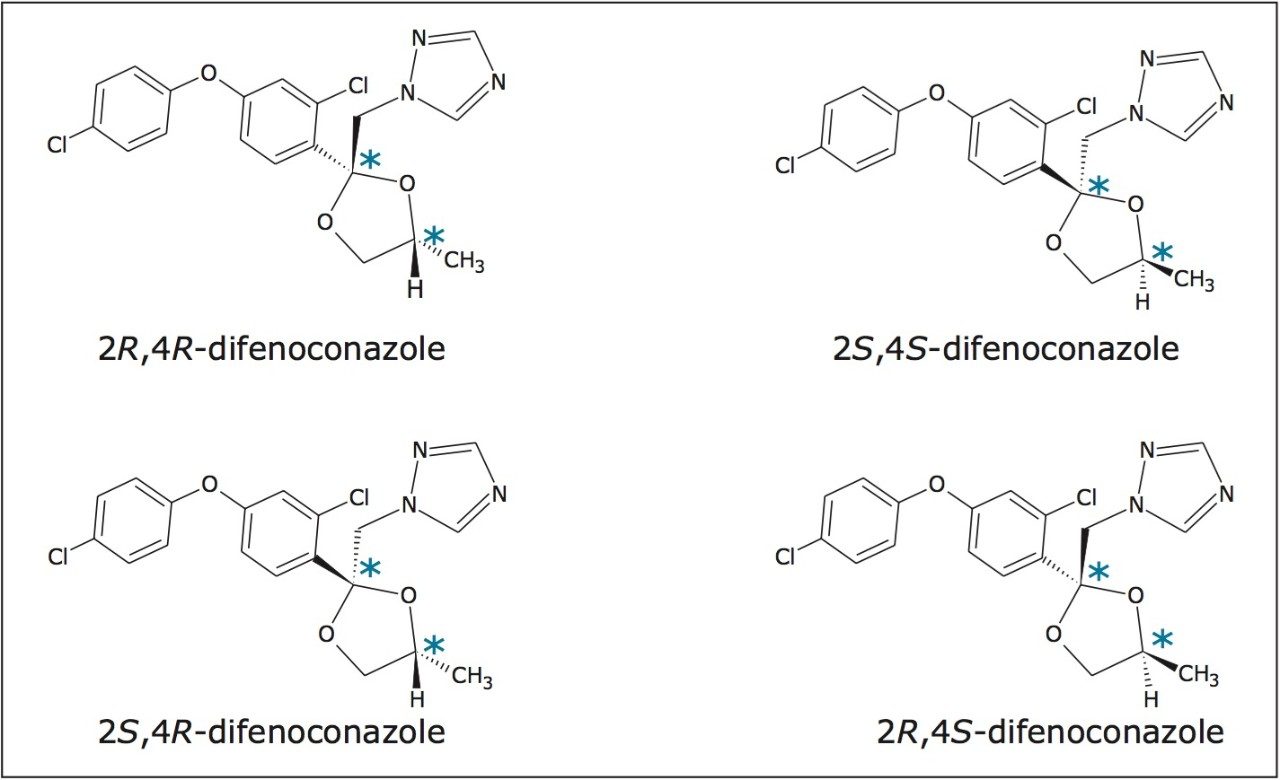
In this application note, we present the enantiomeric and/or diastereomeric separations of three pesticides: metalaxyl-M (a phenylamide fungicide), S-metolachlor (acetanilide class of herbicides), and difenoconazole (a triazole fungicide). Metalaxyl has one chiral center, while metolachlor and difenoconazole have two chiral centers. The structures are shown in Figures 1 to 3. Separations were performed using Waters ACQUITY UltraPerformance Convergence Chromatography System (UPC2). Convergence chromatography is a complimentary separation technique to liquid chromatography, providing orthogonal selectivity and using supercritical CO2 as the primary mobile phase.
Separations were done using the ACQUITY UPC2 System. Detection was by photodiode array (PDA) in combination with single wavelength detection. Empower 3 Software was used for chromatographic data processing.
Sample Preparation: The pesticide standards, supplied by Syngenta, were prepared in 2-propanol.
|
Metalaxyl-M |
|
|---|---|
|
Separation mode: |
Gradient |
|
Column: |
Chiralpak IA-3 4.6 x 150 mm, 3-μm |
|
Co-solvent: |
2-propanol |
|
ABPR: |
2000 psi/138 bar |
|
Flow rate: |
4.0 mL/min |
|
UV detection: |
215 nm |
|
Column temp.: |
55 °C. |
|
Injection volume: |
1 μL |
|
Separation mode: |
Gradient |
|
Column: |
Chiralpak IA-3 4.6 x 150 mm, 3 μm |
|
Co-solvent: |
2-propanol |
|
ABPR: |
2000 psi/138 bar |
|
Flow rate: |
2.5 mL/min |
|
UV detection: |
220 nm |
|
Column temp.: |
35 °C |
|
Injection volume: |
2 μL |
|
Separation mode: |
Isocratic |
|
Column: |
Chiralcel OD-3 4.6 x 150 mm, 3 μm |
|
Co-solvent: |
2-propanol/Butan-1-ol (70/30) |
|
ABPR: |
2000 psi/138 bar |
|
Flow rate: |
2.0 mL/min |
|
UV detection: |
235 nm |
|
Column temp.: |
35 °C |
|
Injection volume: |
2 μL |
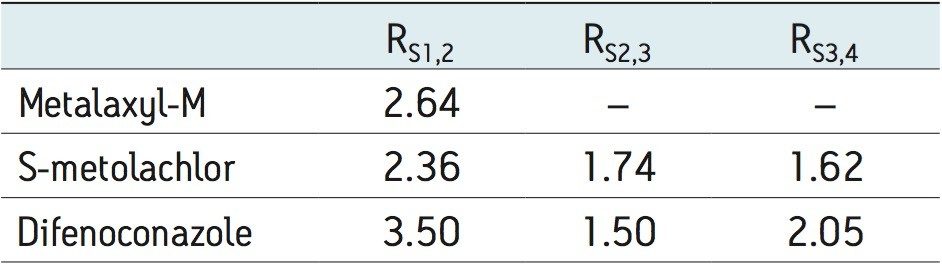
The method development for the standard pesticides began by using a generic screening gradient with a number of chiral columns and co-solvents. This screening step can be completed rapidly due to the shorter analysis times that are possible with this technique. The combination of co-solvent and column that produced the most promising separation for each compound was then optimized. The selectivity in a chiral separation can change markedly by varying the temperature, pressure, and flow rate.8 Gradient and isocratic separations were evaluated; both resulted in successful separation of the stereoisomers for each compound. The separation that showed optimum resolution (Rs) is reported here. The optimized separations for the standard racemic mixture of metalaxyl and the biologically active R-enantiomer,9 metalaxyl-M are shown in Figure 4.
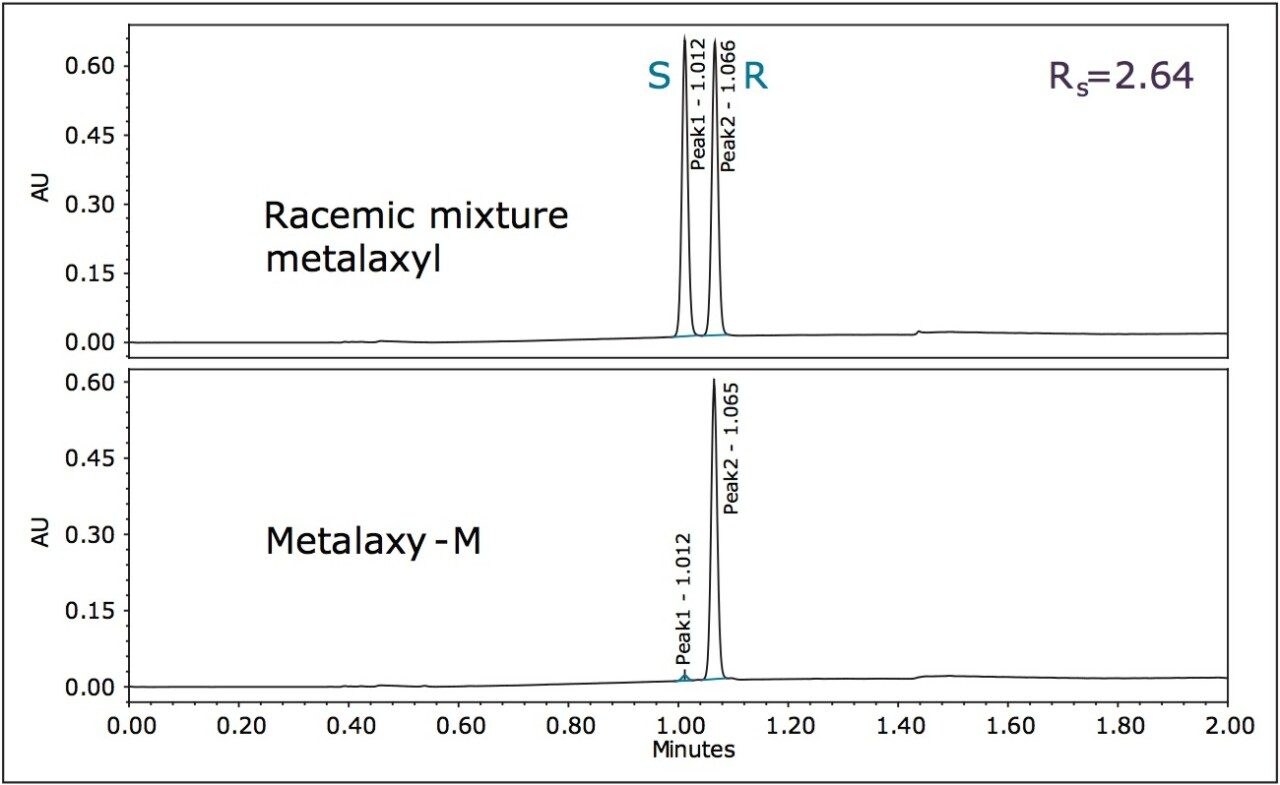
The Rs between the S and R enantiomers was 2.64 (Table 1). A comprehensive survey of enantioselective separations of chiral pesticides in the literature, published in 2009, reported a normal phase isocratic method using hexane/ethanol (60/40) for the resolution of metalaxyl enantiomers. The Rs was reported to be 1.94 with both enantiomers eluting in just under 15 minutes.10,11
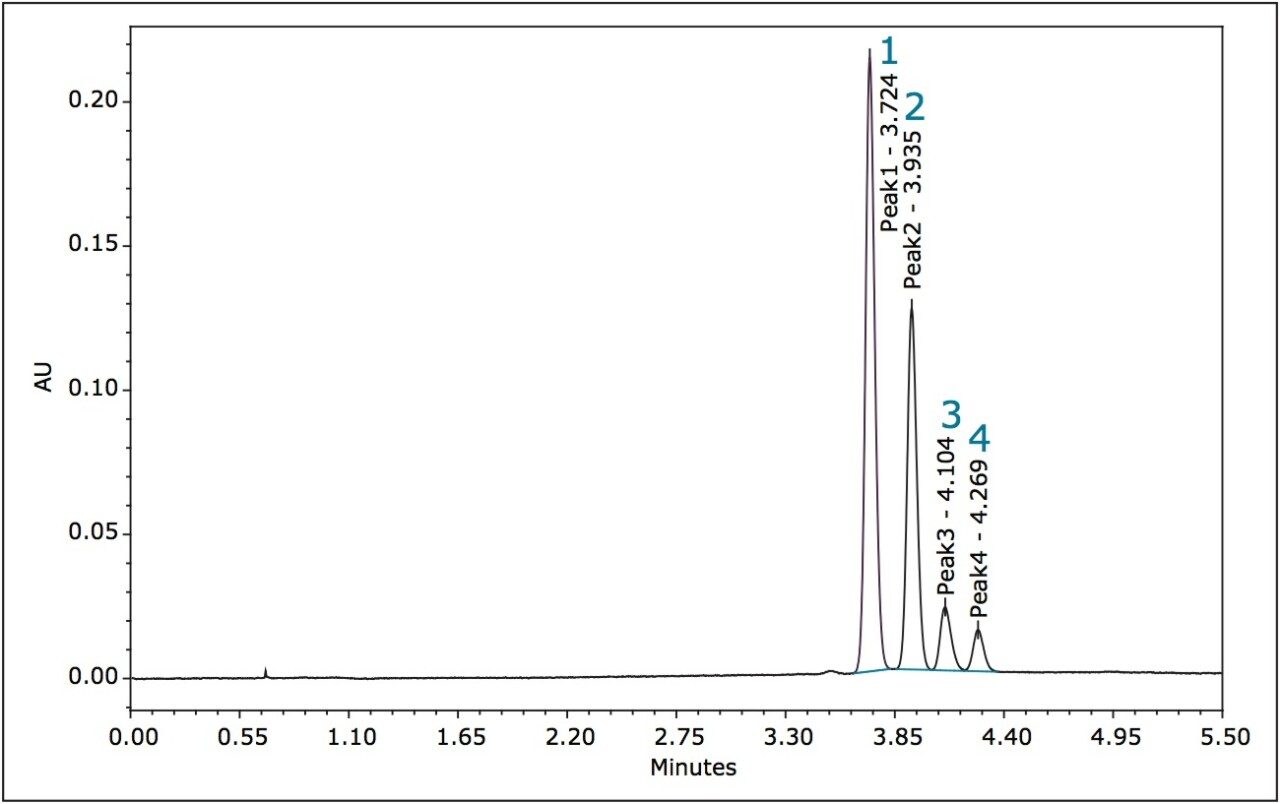
In the case of metolachlor, 95% of the herbicidal activity is reported to come from the S pair of enantiomers.12,13 The separation of all four stereoisomers of metolachlor is shown in Figure 5 with the minimum Rs being 1.62 between peaks 3 and 4, (Table 1). The baseline resolution of the stereoisomers of metolachlor using a normalphase separation with hexane/diethyl ether (91/9) was also reported in the 2009 article. A figure from this publication showed all four stereoisomers eluting between 20 to 30 min.10,14 The ACQUITY UPC2 methods reported here have significantly shorter run times for both metalaxyl and metolachlor. In addition, potentially hazardous solvents are not required.
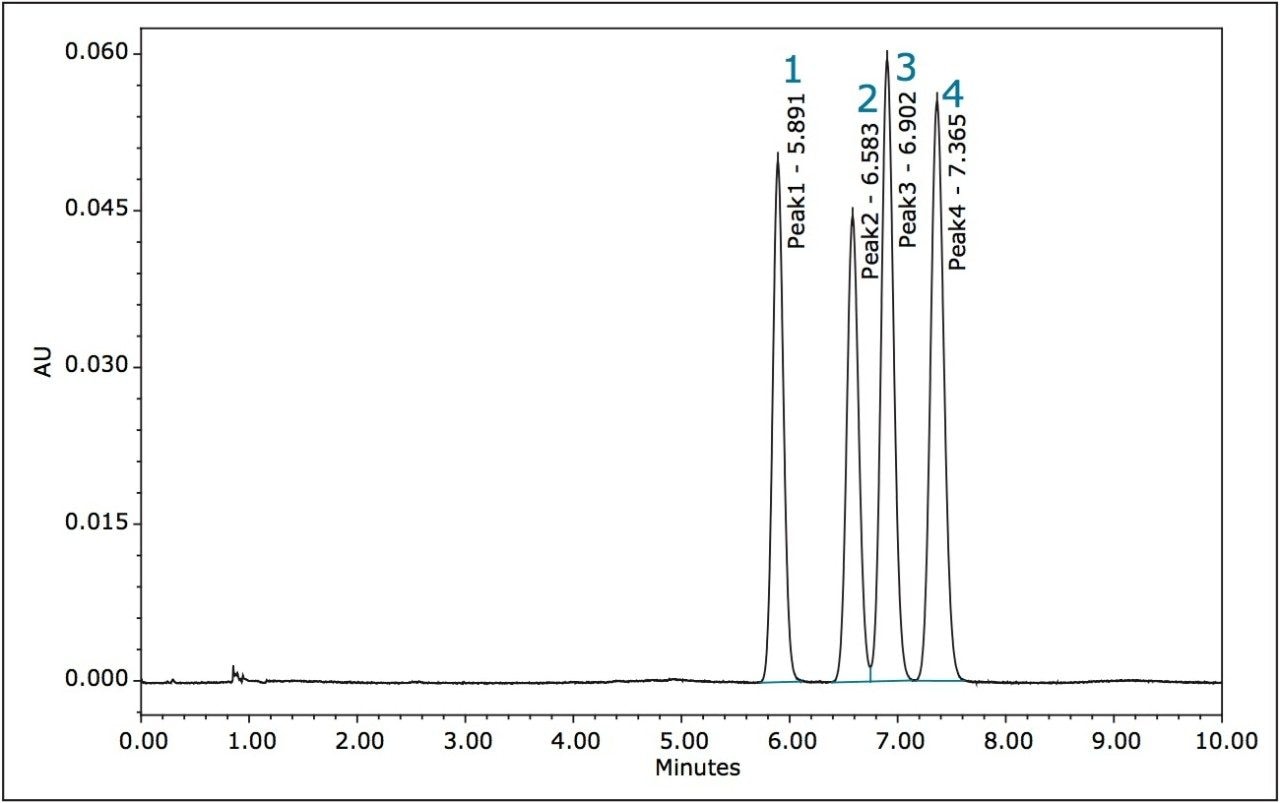
The separation of the stereoisomers in a racemic mixture of difenoconazole is shown in Figure 6. The minimum Rs of 1.5 was between peaks 2 and 3. A normal-phase separation of the stereoisomers of difenoconazole using hexane/ethanol (90/10) was reported in the previously mentioned paper, resolution of the four stereoisomers took place between 30 and 55 minutes. Baseline separation (Rs>1.5) was not achieved between peaks 1,2 or 2,3 in the reported method.10,15
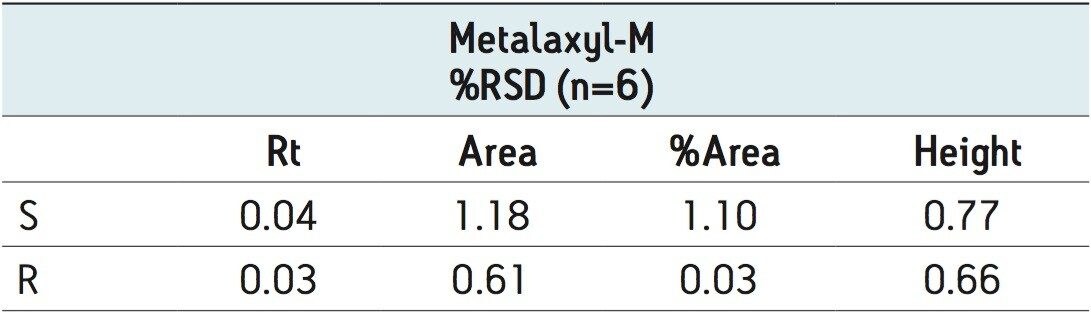
The optimized ACQUITY UPC2 methods allowed increased sample throughput and improved enantiomeric and diastereomeric resolution. In the case of metalaxyl-M (Figure 4), both enantiomers eluted in one minute; for S-metolachlor all four stereoisomers had eluted in 4.5 minutes (Figure 5). The resolution of the four difenoconazole stereoisomers used isocratic elution in under eight minutes (Figure 6), six times faster than some normal-phase methods reported in the literature. Reproducibility data (n=6) for retention time, area, area %, and height gave % RSD’s less than or equal to 1.22 for all the stereoisomers of each compound, (Tables 2 to 4).
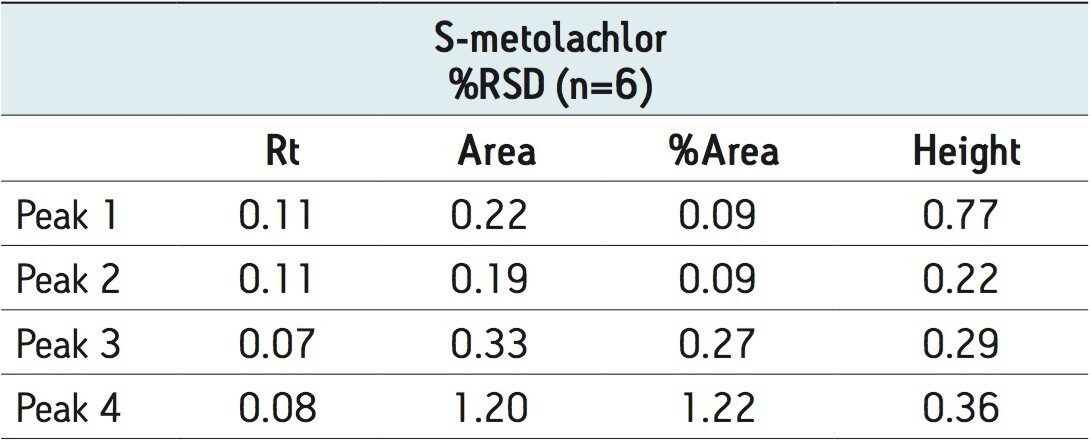
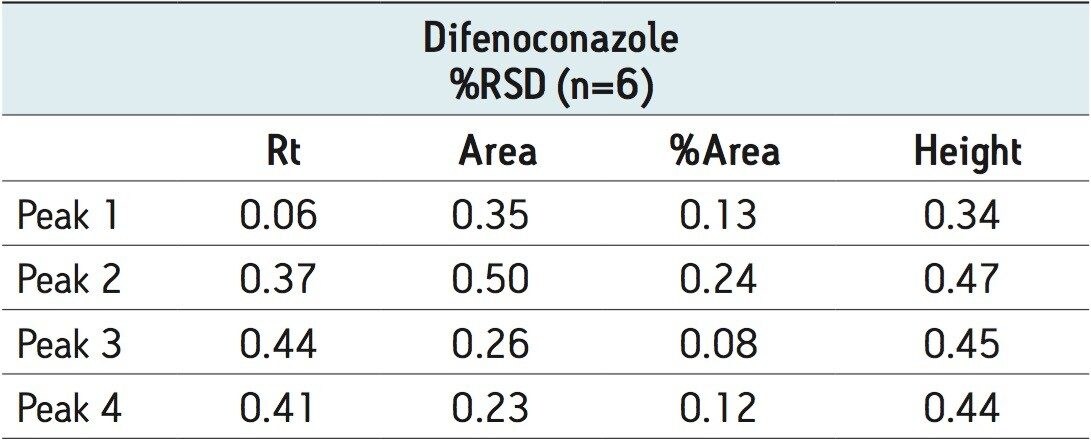
In this application note we have shown the analysis of chiral pesticides using ACQUITY UPC2. ACQUITY UPC2 allows high efficiency separations that can significantly increase the sample throughput when compared with traditional normal-phase separations.10 The time taken to develop a method from the column and co-solvent screening step to the final optimized method is decreased. The methods described here use supercritical CO2 as the primary mobile phase and predominantly 2-propanol as the organic modifier. The need to use large volumes of potentially hazardous solvents is reduced as is the cost associated with solvent waste disposal. The %RSD’s obtained were comparable to those obtained by UPLC/UV methods.
The study of enantioselective toxicity and environmental fate has previously been a challenge due to the difficulty in resolving chiral compounds. The benefit of having faster analytical methods to resolve chiral compounds means that critical information pertaining to their stereoselective behavior can be obtained more rapidly.
720004657, May 2013