
This application note demonstrates the conversion of the USP method to convergence chromatography. The use of supercritical carbon dioxide (CO2) as a mobile phase, combined with modifiers and additives, allows for a robust quantitative alternate method to GC with improved sample stability and simplified sample preparation.
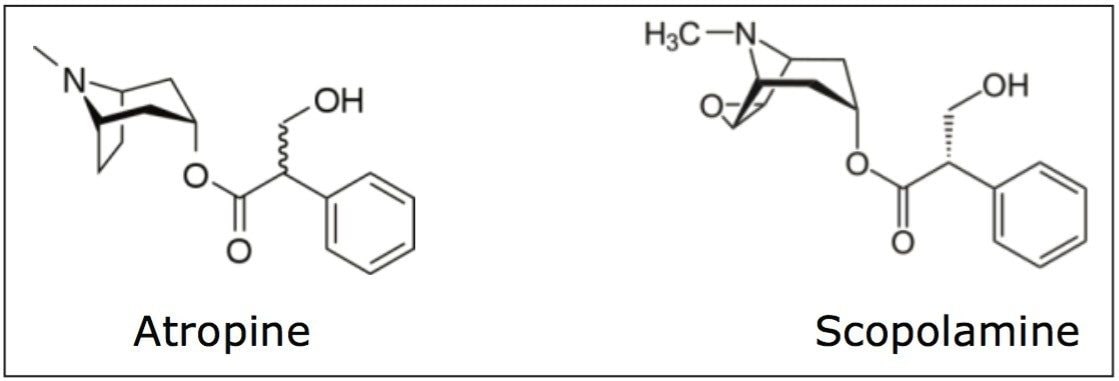
The extract of Atropa belladonna, a perennial native to south and central Europe, North Africa, and East Asia has been used for centuries to treat a number of medical conditions, including ulcers and other gastrointestinal disorders, and pain. It is also used as an anti-muscarinic agent. The extract is primarily composed of the tropane alkaloids, scopolamine and hyoscyamine (atropine), which are not only effective medicinal agents but are also highly toxic. This dichotomy makes quantification of these alkaloids in extracts critical for safety.
The current United States Pharmacopoeial Convention (USP) method for Atropa belladonna extract specifies a gas chromatography (GC) method, using a 1.2 x 4.0 mm glass column packed with 3% G3 on S1AB with helium as a carrier gas. The use of highly volatile diluent dichloromethane is required for sample preparation. While direct injection is typically performed for these analyses, the high temperature required for GC analyses can lead to dehydration and conversion of scopolamine and atropine to their apo-forms.1 Often, these issues are resolved by derivatization.2 This application demonstrates the conversion of the USP method to convergence chromatography. The use of supercritical carbon dioxide (CO2) as a mobile phase, combined with modifiers and additives, allows for a robust quantitative alternate method to GC with improved sample stability and simplified sample preparation.
Standard solutions of scopolamine hydrobromide (CAS# 114-49-8), atropine free base (CAS# 51-55-8), and homatropine hydrobromide (CAS# 51-56-9) were prepared in 2-propanol. All stock solutions were prepared at 1 mg/mL. Stock solutions were sonicated to ensure dissolution. Calibration standards of scopolamine and atropine were prepared from the stock solutions at 5, 10, 50, 100, 200, and 400 μg/mL with the internal standard (homatropine) at 50 μg/mL.
A commercially available Atropa belladonna extract was purchased. Both the extract and a matrix blank were analyzed. The extract was diluted 1:3 (4x) in 2-propanol. The internal standard (homatropine) was added at 50 μg/mL. The combined solution was filtered, prior to analysis, with a 1-μm glass filter. The matrix blank was spiked with 100 μg/mL of atropine, scopolamine, and internal standard at 50 μg/mL.
|
UPC2 conditions |
||
|---|---|---|
|
System: |
ACQUITY UPC2 with PDA and SQD detection |
|
|
Detection: |
PDA at 220 nm, compensated 410 to 480 nm |
|
|
Vials: |
Clear Maximum Recovery Vials, Screw Neck (p/n 186000327C) |
|
|
Sample filter: |
Acrodisc, syringe filter, glass filter (p/n WAT 200819) |
|
|
Column: |
ACQUITY UPC2 BEH 3.0 x 100 mm, 1.7 μm |
|
|
Column temp.: |
50 °C |
|
|
Sample temp.: |
15 °C |
|
|
ABPR: |
2000 psi (138 bar) |
|
|
Injection volume: |
1 μL |
|
|
Flow rate: |
2 mL/min |
|
|
Mobile phase A: |
CO2 |
|
|
Mobile phase B: |
0.2% ammonium hydroxide (28% to 30%) in 98:2 (v/v) methanol/water |
|
|
Gradient: |
10% to 30% B in 4.5 min, 30% to 40% in 0.5 min, 40% to 5% in 0.5 min |
|
|
Weak needle wash: |
2-propanol |
|
|
Strong needle wash, seal wash: |
Methanol |
|
|
Conditioning parameters: |
1 mL/min, 100% mobile phase B |
|
Mass Spectrometer: |
ACQUITY SQD with PCM II and 515 Pump for makeup flow |
|
Splitter: |
UPC2 MS splitter |
|
Ionization mode: |
ESI+ |
|
Acquisition range: |
70 to 500 m/z |
|
Capillary voltage: |
3 |
|
Cone voltage: |
30 |
|
Desolvation gas: |
500 |
|
Makeup flow: |
0.4 mL/min methanol |
|
Data management: |
Empower 3 Software |
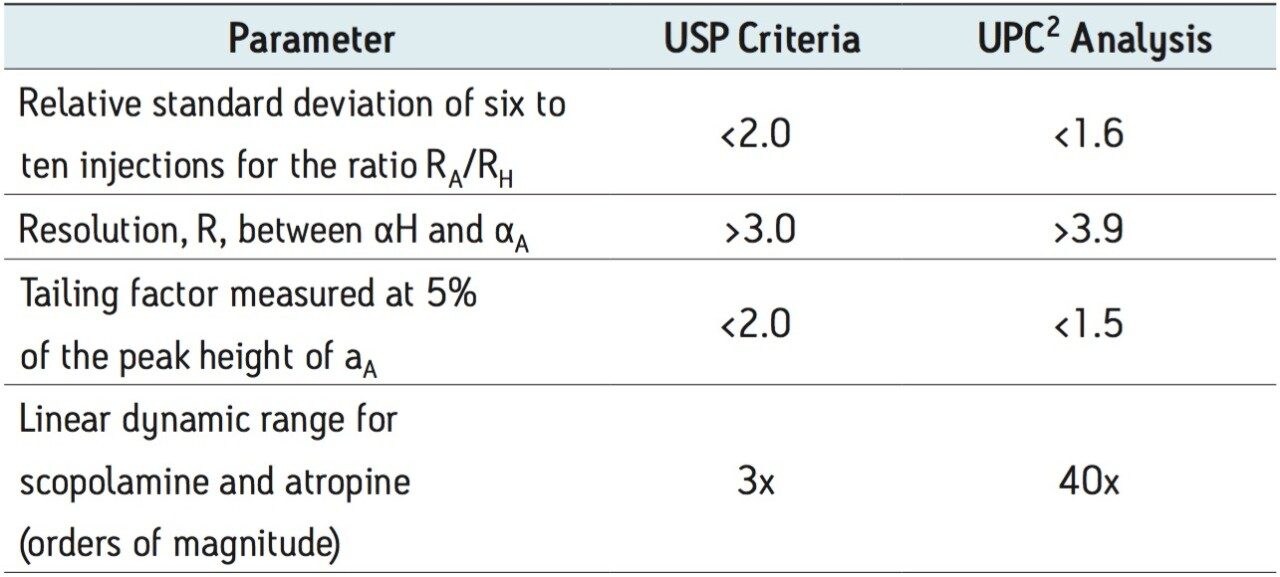
The analysis of Atropa belladonna extract by GC has been well documented.2 The USP methodology requires the use of GC for quantification of scopolamine and atropine. The assay suitability requirements include the following:
In order to demonstrate equivalence of the UPC2 method, these criteria as well as robustness were evaluated.
UltraPerformance Convergence Chromatography (UPC2) is a separation technique that uses supercritical CO2 as the primary mobile phase. For highly basic compounds, modifiers containing basic additives are required to minimize secondary interactions and improve peak shape.4 For the analysis of atropine (basic) and scopolamine (neutral), initial screening was performed on a number of different stationary phases using methanol as a co-solvent or modifier. As expected, poor peak shape was observed for atropine. The addition of basic additives improved peak shape to satisfy the tailing factor requirement in the USP method. Ammonium hydroxide was selected as the additive of choice because of its compatibility with mass spectrometry. Water was used as a second additive to further improve peak shape and reproducibility. The resulting method, on an ethylenebridged hybrid (BEH) stationary phase, met all of the USP suitability requirements, shown in Table 1, as well as allowing for a high throughput method in less than 5 minutes. Prior to suitability testing the column was also conditioned for 2.5 hours or 200 column volumes of the modifier-additive.
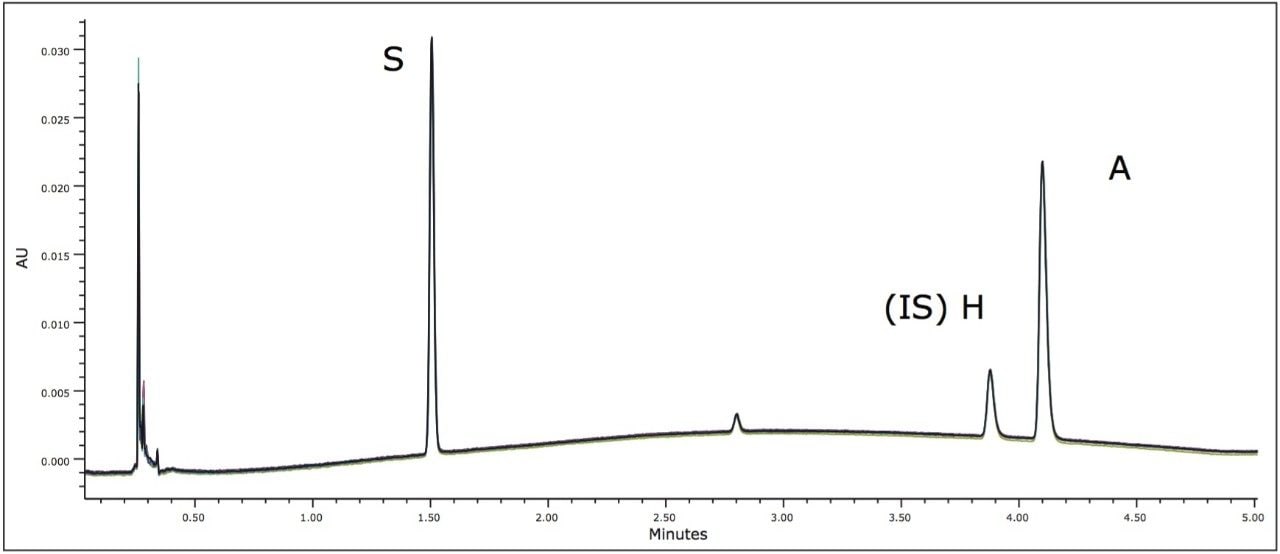
In order to meet the USP suitability requirements and evaluate reproducibility, eight replicate injections were performed for each calibration standard. Retention time reproducibility was within 0.05% RSD for each standard. Peak areas relative to the internal standard (H) were less than 1.6% for both scopolamine and atropine, meeting USP suitability requirements. The standard chromatograms also displayed a peak for bromide at approximately 2.8 minutes.
Based on the standard described in the USP method, the requirements for the linear dynamic range are equivalent to 32 to 120 μg/mL for scopolamine and 320 to 1200 μg/mL for atropine. Conversion to UPC2 required a shift to lower levels for atropine due to mass overloading above 400 μg/mL; however, the overall linear dynamic range was exceeded for both components (40x versus 3x per component). Linearity was achieved from 10 to 400 μg/mL (0.01% to 0.4%) for both scopolamine and atropine, as shown in Figure 3 and Table 2. Overlay chromatograms of the complete set of calibration standards were evaluated, as shown in Figure 3. There was no significant distortion that compromised quantification of atropine or scopolamine over the tested range of concentrations. The detection levels are within the linearity specifications for the detector. These characteristics indicate that the qualitative properties of the assays are maintained over this concentration range.

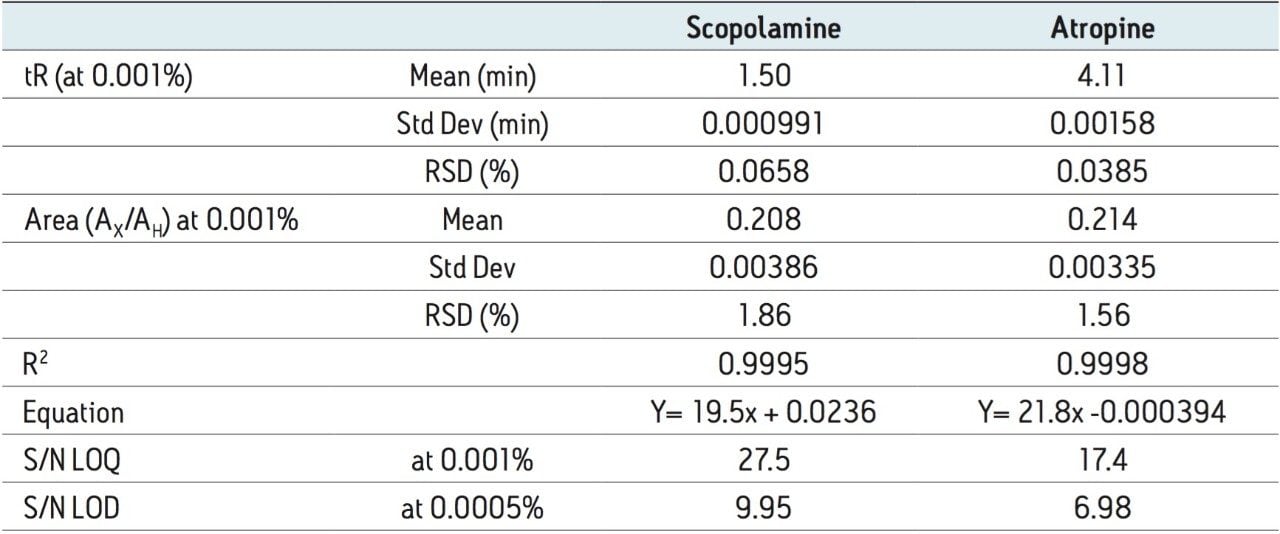
The resulting convergence chromatography method met all of the suitability requirements specified for the USP method. Peak area reproducibility was calculated relative to the internal standard (homatropine), where Ratio(A) = Area(A)/Area(H). The area ratio %RSD for scopolamine and atropine were within the suitability guidelines (<2%). The linear dynamic range met all specifications with R2 > 0.999, deviation <10% for points at the limit of quantitation (LOQ) and <5% for all other points in the calibration curve. The USP signal-to-noise (S/N) ratio was >10 at 10 μg/mL which was the LOQ. The limit of detection (LOD), defined as USP S/N >3.0, was 5 μg/mL for atropine and <5 μg/mL for scopolamine. These results are summarized in Table 2.
While the USP method outlines the procedure for extraction of Atropa belladonna leaves, because of limited availability commercial Atropa belladonna extract was substituted and analyzed using the newly developed UPC2 method. For equivalency of the UPC2 method compared to the USP GC method, dilution of the extract by a factor of 4 was required. The extract was diluted in 2-propanol to minimize peak distortion. The internal standard (homatropine) was added to extract, and filtration of the diluted sample was required to remove particulates that formed with the addition of the diluent. The diluted extract contained 49.25 μg/mL atropine or 197 μg/mL in the commercial preparation. Scopolamine was present at levels below the LOQ of both the UPC2 and USP GC method. To ensure accurate quantification of the extract, a matrix blank was spiked with scopolamine and atropine. Recoveries of 105% and 110%, respectively, were observed.
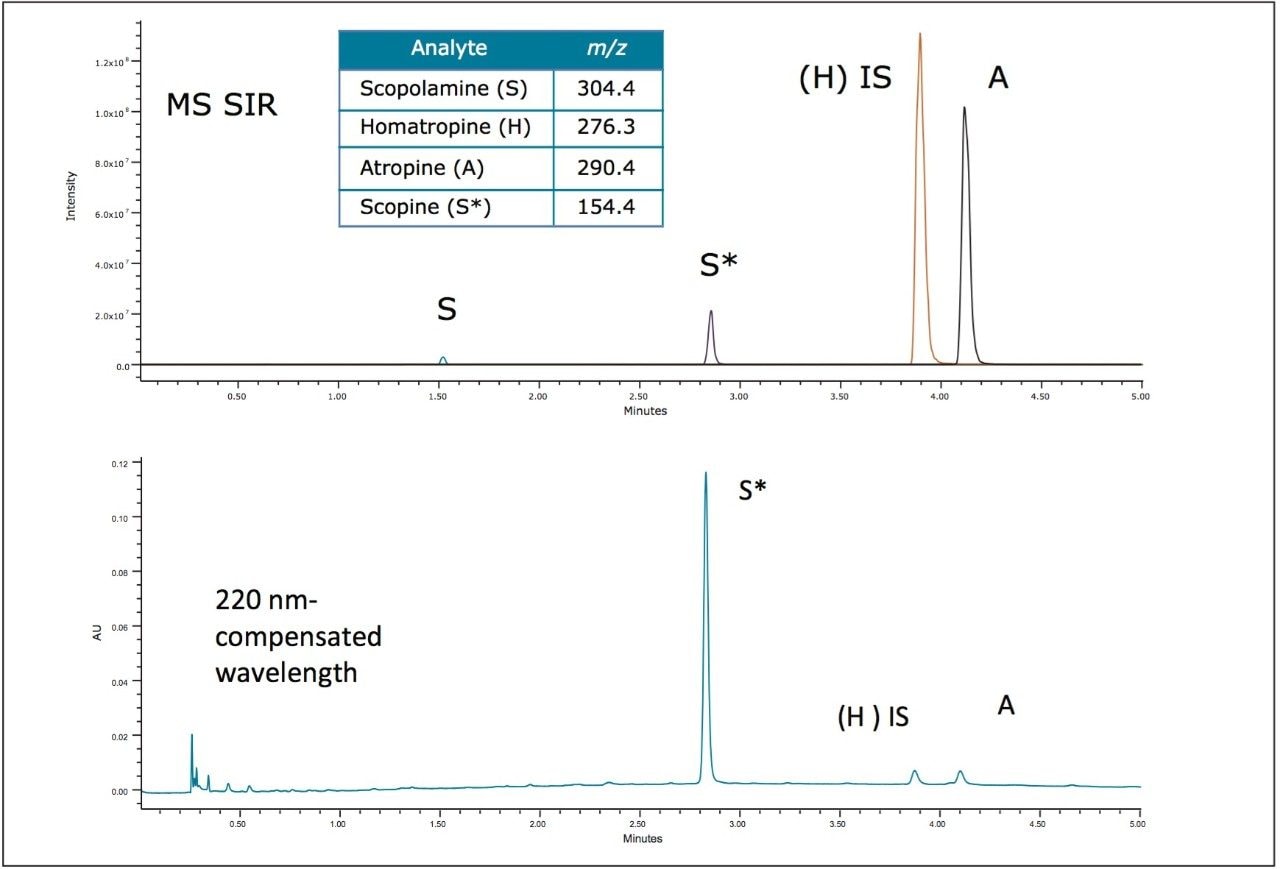
Mass spectrometry (MS) was used for confirmation of the analytes of interest as well as identification of any unknown components in the extract. While the analysis of the Atropa belladonna extract by UV indicated very low amounts of quantifiable scopolamine, single ion recording (SIR) in MS revealed the presence of scopolamine in the extract at levels below the LOD of the UV method, as shown in Figure 4A. In addition, a peak at 2.8 min was present in the extract but not observed in the calibration standards. The mass spectrum indicated the peak (m/z 154.4) is consistent with the mass of scopine which is likely formed by the hydrolysis of scopolamine.5
Convergence chromatography provides an alternative technique for the analysis of highly polar compounds that have been traditionally analyzed by GC, thereby simplifying sample preparation by eliminating the need for derivatization.2 As described in this study, the use of supercritical CO2 provides a reproducible, high throughput analysis for tropane alkaloids while meeting USP suitability guidelines. Furthermore, the robustness of the method illustrates the ability to develop reliable, quantitative methods using convergence chromatography.
720004573, January 2013