This application note focuses on the analysis of extractables in the pharmaceutical and food packaging industries.
The ACQUITY UPC2 System streamlines the analytical workflow by providing flexibility with various common solvent systems. The MV-10 ASFE System represents a substantial savings in solvent consumption and run time as well as greater flexibility than other extraction techniques.
Analysis of extractables in the pharmaceutical and food packaging industries is well established.1-3 Analytical workflows can incorporate various techniques. Similarly, the evaluation of container closure systems can include various extraction techniques. The ACQUITY UPC2 System streamlines the analytical workflow by providing flexibility with various common solvent systems resulting from extraction procedures.4 While supercritical fluid plays a key role in improving analytical workflow, the question is raised: “Can the sample extraction process be streamlined to utilize one technique, namely a supercritical extraction process?”
Several techniques can be used to prepare sample extracts in the extractables analysis process. Typically, either a Soxhlet, microwave, or supercritical fluid extraction (SFE) are performed. The extraction solvents must cover a wide range of polarities to ensure that non-polar and polar analytes are extracted from packaging material. The Soxhlet apparatus can be a very attractive option due to its relatively inexpensive setup. However, when the price of extraction solvents and their waste disposal is considered, microwave and SFE offer cost saving benefits including reduced solvent consumption and waste disposal, as well as valuable reduction in analysis time.
In this application, four different types of packaging material were extracted including: high density polypropylene pill bottle (HDPE), low density polypropylene bottle (LDPE), ethylene vinyl-acetate plasma bag (EVA), and polyvinyl chloride blister pack (PVC). Following extraction, the resulting solutions were rapidly screened for 14 common polymer additives using an UltraPerformance Convergence Chromatography (UPC2) System with PDA and single quadrupole (SQD) mass detection. Microwave and Soxhlet were used to separately prepare IPA and hexane extracts, while different concentrations of IPA were used as the co-solvent for SFE extractions. Here, the extraction profiles of the different techniques are compared.
|
UPC2 Conditions |
|
|---|---|
|
System: |
ACQUITY UPC2 with PDA and SQD Detection |
|
Column: |
ACQUITY UPC2 BEH 2-EP 3.0 x 100 mm, 1.7 μm |
|
Modifier: |
1:1 methanol/acetonitrile |
|
Flow rate: |
2 mL/min |
|
Gradient: |
1% B for 1 min, to 20% over 2.5 min, hold for 30 s, re-equilibrate back to 1% |
|
Column temp.: |
65 °C |
|
APBR: |
1800 psi |
|
Injection volume: |
1.0 μL |
|
Run time: |
5.1 min |
|
Wavelength: |
220 nm |
|
MS scan range: |
200 to 1200 m/z |
|
Capillary: |
3 kV |
|
Cone: |
25 V |
|
Make-up flow: |
0.1% formic acid in methanol, 0.2 mL/min |
|
Data management: |
Empower 3 Software |
The samples of HDPE, LDPE, EVA, and PVC (2 g) were cut into 1x1 cm pieces and subsequently extracted in either 10 mL of isopropanol or 10 mL of hexane for 3 h at 50 °C.
Soxhlet extractions were performed by placing cut pieces (roughly 1x1 cm) of material (3 g for PVC, 5 g for HDPE, LDPE, or EVA) into a Whatman 33 x 94 mm cellulose extraction thimble. The thimble was then placed in a conventional Soxhlet extraction apparatus, consisting of a condenser, a Soxhlet chamber, and an extraction flask. Approximately 175 mL of extraction solvent (either hexane or IPA) was added into the Soxhlet apparatus. All samples were extracted with the hot boiling solvent mixture for 8 h. Upon completion, the extraction solvent was reduced to near dryness and reconstituted in 15 mL of either hexane or IPA. Prior to analysis, extracts were filtered through a 0.45-µm glass fiber syringe tip filter to remove any particulates.
Supercritical fluid extraction (SFE) was performed using a Waters MV-10 ASFE System. For each SFE experiment, cut pieces (roughly 1x1 cm) of material were loaded into 10-mL stainless steel extraction vessels (2 g for PVC, 3 g for HDPE, LDPE, or EVA). Two distinct extractions were performed on each material. The first used 5.0 mL/min carbon dioxide plus 0.10 mL/min IPA, the second used 4.0 mL/min carbon dioxide plus 1.0 mL/min IPA. All extractions were performed at 50 °C and 300 bar back pressure using a 30-min dynamic, 20-min static, and 10-min dynamic program that was repeated twice. IPA was used as a makeup solvent at 0.25 mL/min. For high IPA extractions, following the extraction process, collected solvent (a mixture of the co-solvent and make-up solvent) was reduced to near dryness and reconstituted in IPA (10 mL for PVC, 9 mL for HDPE, LDPE, and EVA). For low IPA extractions, the collected solvent was brought up to volume accordingly. Prior to analysis, extracts were filtered through a 0.45-µm glass fiber syringe tip filter to remove any particulates. Total extraction time per sample was 2 h.
Comparing the duration of the extraction processes, Soxhlet extracted each sample individually for 8 h. Microwave could accommodate up to 16 samples simultaneously over a 3-hour extraction. The SFE process took 2 hours per sample with up to 10 samples loaded onto the sample tray. Even if more Soxhlet apparatus were used simultaneously, the total extraction time would still significantly exceed microwave or SFE extraction times.
In terms of solvent usage, Soxhlet required up to 175 mL of solvent, followed by evaporation to reduce sample volume. Microwave used 10 mL of solvent that could be dried down if improvements in sensitivity are needed. SFE offered the greatest flexibility in sample pre-concentration. Under low IPA extraction conditions, the final volume collected was approximately 5 mL, and brought up to volume to have the concentration of the sample comparable to microwave and Soxhlet samples. Under high IPA extraction conditions, the total volume collected was ~30 mL, which had to be evaporated to obtain the final concentration.
The fewest number of extractables were observed in the PVC and EVA samples analyzed after microwave extraction. The most extractables were observed using either hexane or IPA extract in the LDPE sample, as shown in Figure 1.
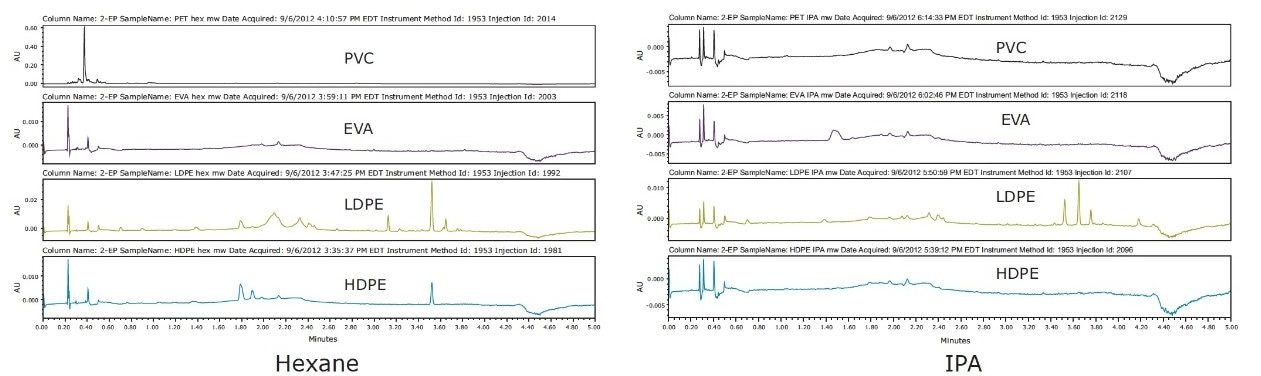
Using Soxhlet extraction, several additional peaks were observed in the PVC chromatograms, as shown in Figure 2, which were not visible following microwave extraction. The observable differences are possibly due to the longer extraction times and higher extraction temperature used in Soxhlet extraction.

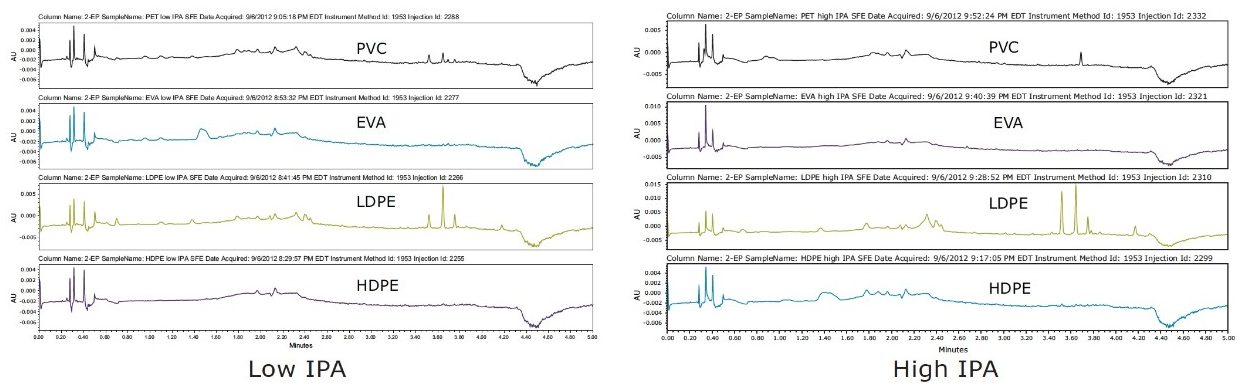
Visually comparing SFE extraction profiles with the other two techniques, SFE extracted similar amounts of analytes as Soxhlet, and a greater amount than microwave extraction of PVC, as shown in Figure 3. High IPA extracted higher amounts in LDPE than the lower percentage in the IPA extraction experiment. This illustrated the flexibility and ease of adjusting to determine the optimal percentage of modifier needed for each plastic material to achieve a successful extractables analysis.
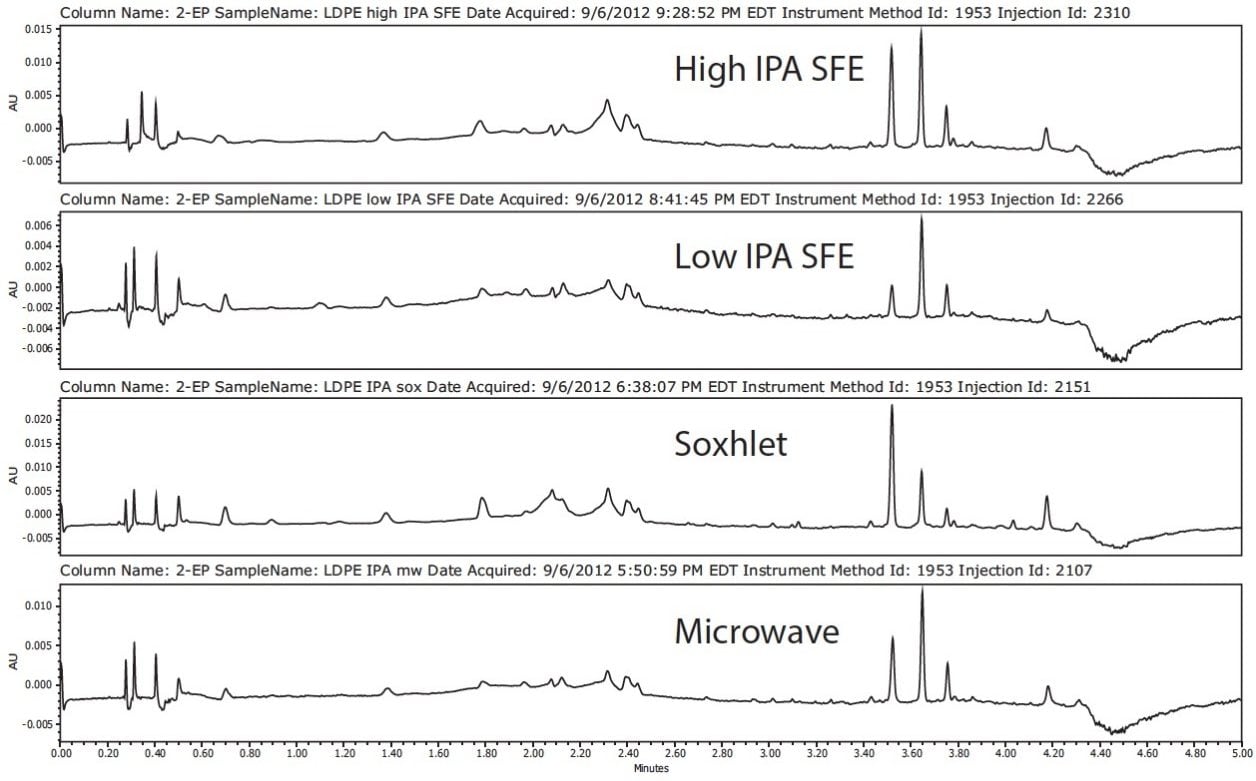
All extraction techniques using IPA as the solvent produced similar chromatographic profiles for the LDPE sample, as seen in Figure 4. Concentration of the extractables can be increased by extended extraction times, higher temperature in microwave and Soxhlet extractions, or a higher level of IPA in the case of SFE. Hexane extractions were not performed by SFE since CO2 is a non-polar solvent with similar chemical properties to hexane; therefore, comparable results were expected.
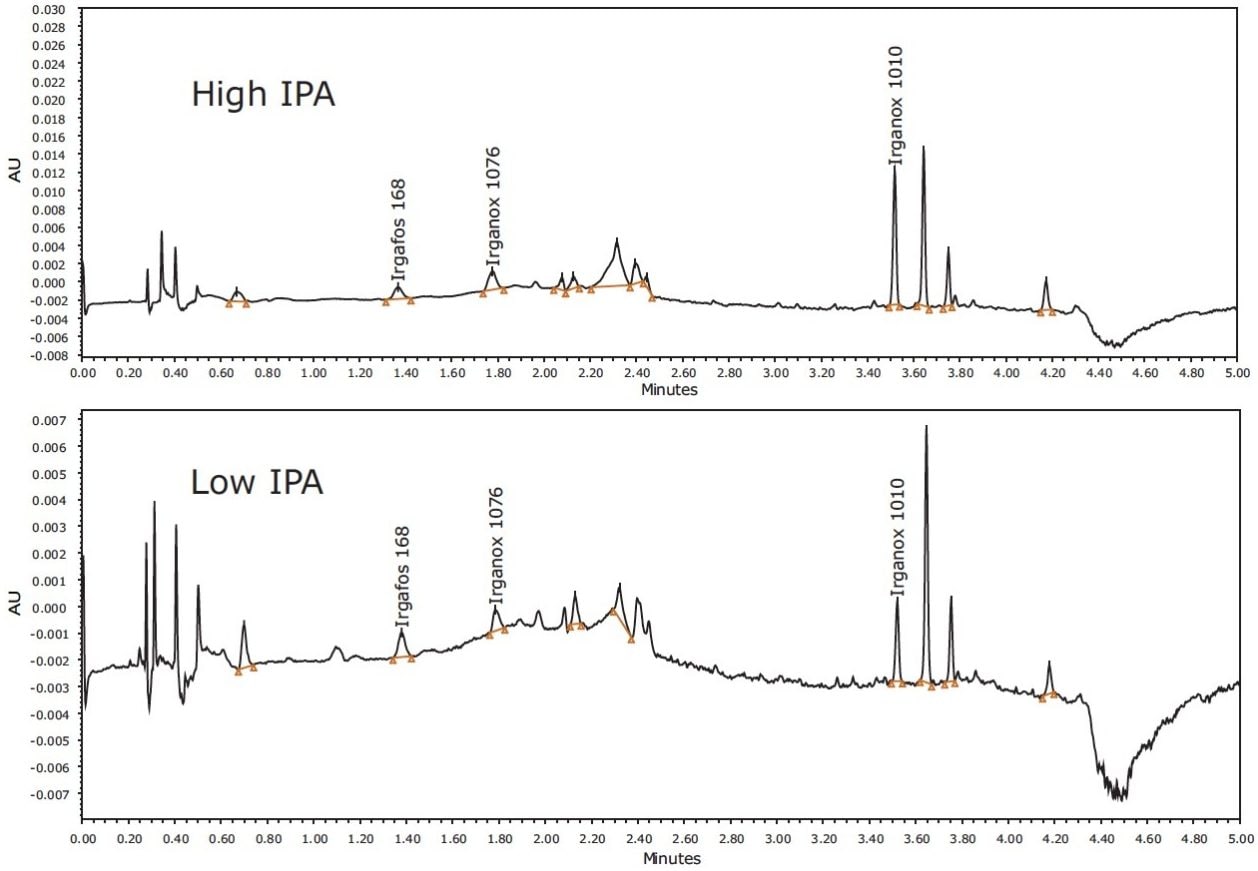
Examples of identified compounds in LDPE hexane extracts are shown in Figure 5.
In summary, all of the techniques are comparable in terms of types of compounds extracted. However, it was determined that SFE offers many advantages over other extraction techniques when time and resources are important. The MV-10 ASFE System is software controlled, providing automated method development. There can be up to four co-solvents available for use, and various percentages and extraction times can be set in the methods. Soxhlet and microwave require manual solvent changes for each step in method development, which is quite time-consuming when conducting a quality by design (QbD) study.
SFE provided 80% to 97% savings in solvent consumption, and a 75% savings in extraction time compared to Soxhlet extraction. The software controlling SFE allowed automated method development to determine the optimal percentages and choices of extraction co-solvent. In addition, SFE provided flexibility in sample pre-concentration compared to microwave extraction.
720004509, November 2012