
This application note describes the advantages of employing the data management and reporting capabilities of NuGenesis SDMS during an impurity profiling project.
The objective of impurity profiling is to identify and quantitate impurities that are present in an API or drug product. Impurities may take the form of three broadclassifications: (1) organic impurities, (2) inorganic impurities, and (3) residual solvent impurities. Organic impurities typically arise from the manufacturing process and may include unreacted starting materials, reaction intermediates, degradation products, and reaction by-products. Some of these impurities may even be genotoxic. Inorganic impurities include ligands and catalysts, heavy metals, inorganic salts, and filter aids. Residual solvents can be either organic or inorganic solvents used during manufacturing.
The U.S. FDA and other regulatory agencies require identification and quantitation of impurities above specific levels. Hence, conducting impurity profiling projects requires thorough documentation, robust data management, and the use of a variety of analytical techniques, e.g., LC/UV, LC-MS, and NMR, in order to provide prove that the impurities have been properly characterized.
With the variety of techniques used and the complexity of the data and reports generated, the scientists and management involved need a robust documentation system to systematically store and catalog the data and then combine the data and results into suitable reports. This laborious and potentially error-prone task is often performed manually due to the vastly different data formats the analytical instruments generate.
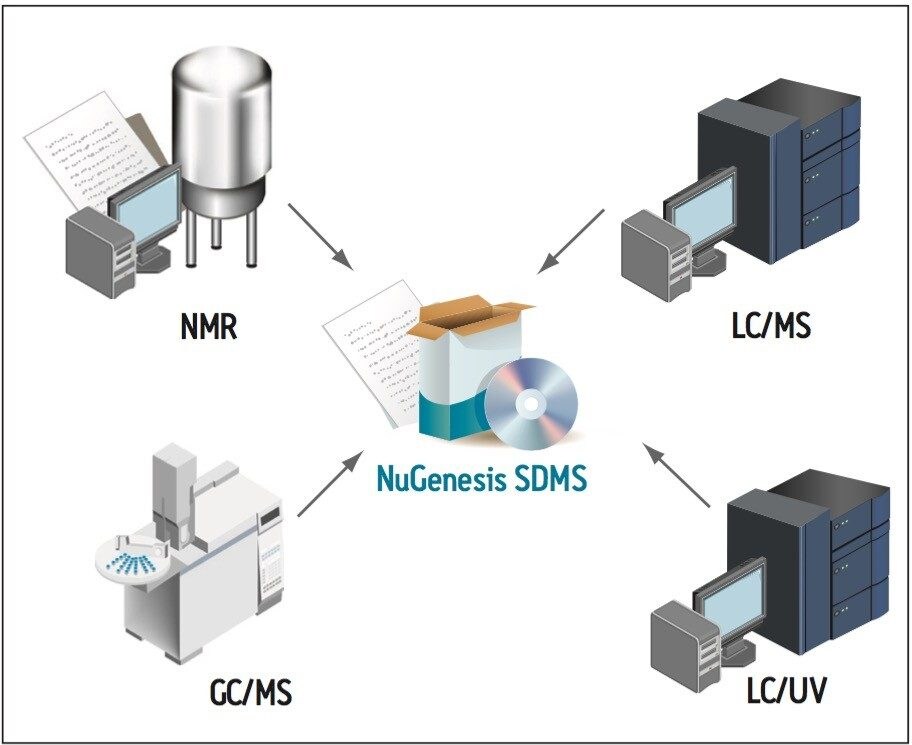
The Waters NuGenesis Scientific Data Management System (SDMS) can automatically capture and catalog analytical data produced during an impurity profiling project into a centralized data repository (Figure 1). The system captures both raw analytical data and printed test reports from all laboratory instruments used during an impurity profiling project, e.g., LC/UV, LC-MS, NMR, and GC-MS. Capturing data within a centralized repository aids data review and approval, streamlines report creation, and promotes interdisciplinary collaboration.
In addition to data collection and archiving functionality, NuGenesis SDMS includes an analytical Electronic Laboratory Notebook (ELN) called SDMS Vision Publisher. SDMS Vision Publisher gives scientists and management the ability to quickly and easily create reports in many formats.
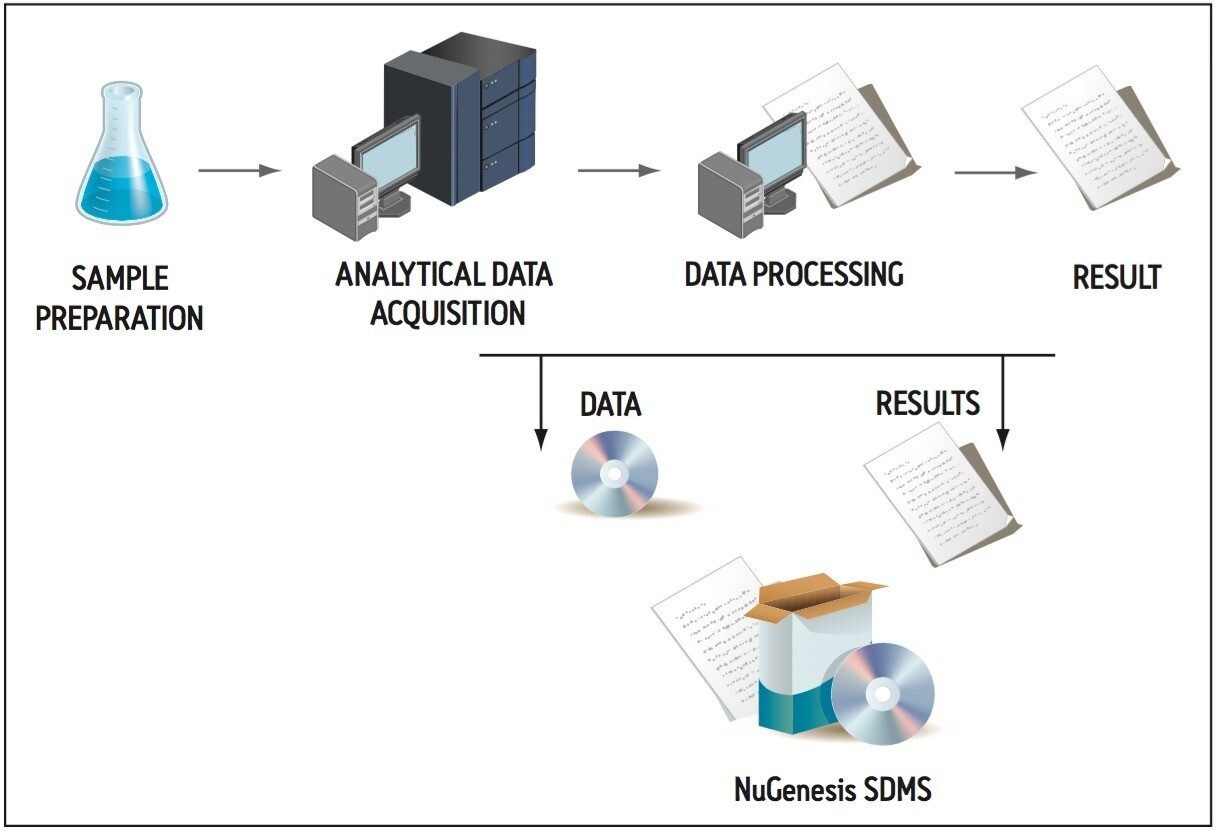
A typical impurity profiling experiment may take place using four key steps (Figure 2). First, a sample is collected and prepared for analysis. Second, the sample is analyzed by using various analytical instruments such as LC-MS or NMR. Third, the raw data created by the instrument is processed using the relevant software application to provide information that will assist with identification and quantification. Fourth, a printed test result is created to summarize the instrumental results. Subsequently, this impurity profiling workflow can generate four different types of data:
In many analytical laboratories, data management tasks are typically the responsibility of laboratory personnel. With NuGenesis SDMS, electronic and printed raw data and reports can be automatically captured and cataloged in the SDMS database (Figure 2). This level of automation can significantly reduce the time and effort associated with data acquisition, processing, reporting, and archiving.
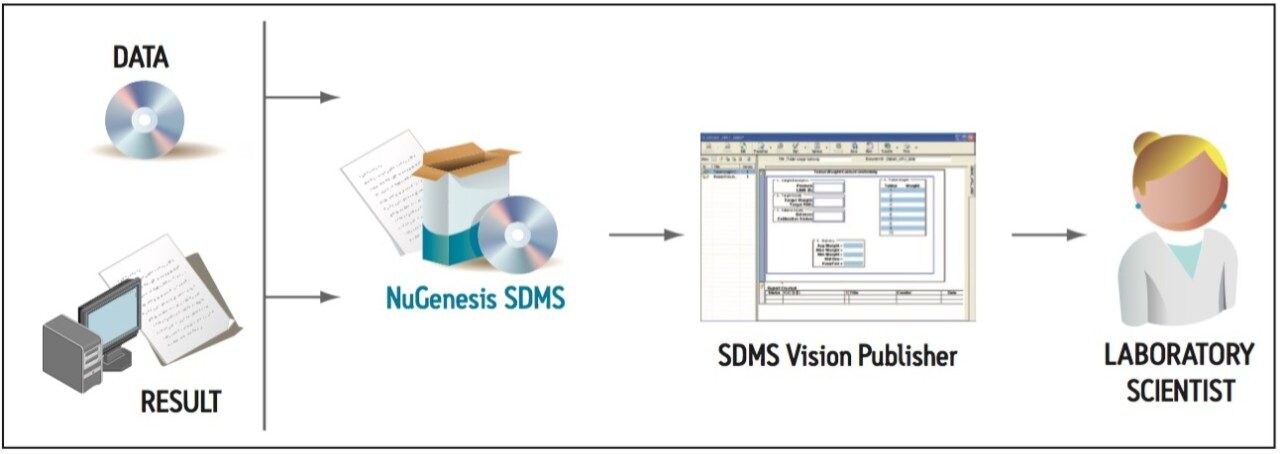
Capturing and cataloging diverse analytical data (such as that generated during an impurity profiling project) into NuGenesis SDMS enables centralized data storage and standardized data format. Then, SDMS Vision Publisher, acting as a portal into the SDMS data repository, streamlines impurity report creation by providing capabilities to combine diverse data and analytical reports into one seamless summary report. The documentation workflow from NuGenesis SDMS to SDMS Vision Publisher is shown in Figure 3.
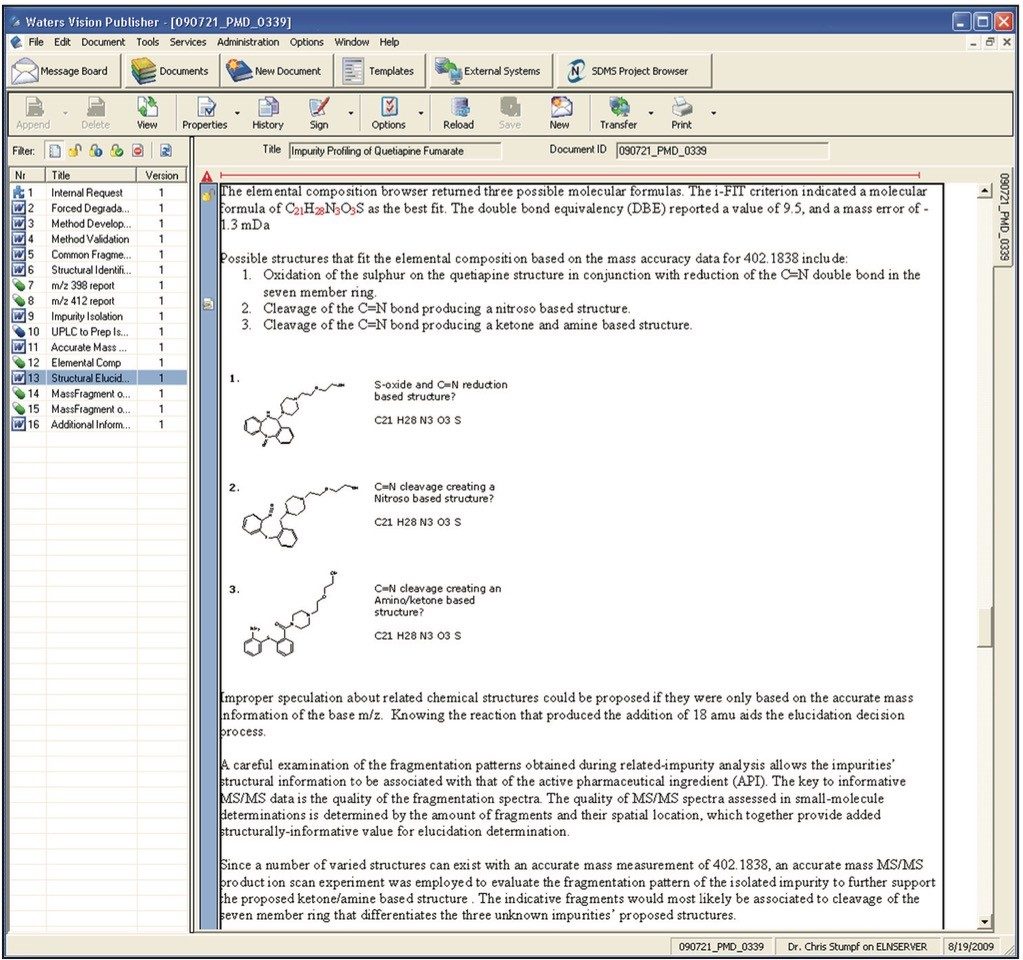
For example, a typical summary report (which may consist of comments, observations as well as chromatograms, spectra, tables, etc.) is shown in Figure 4.
In addition, it is possible to directly incorporate a precursor ion scan (LC-MS/MS) test result from within the integrated NuGenesis SDMS data repository into the impurity profiling report. This is shown in Figure 5.
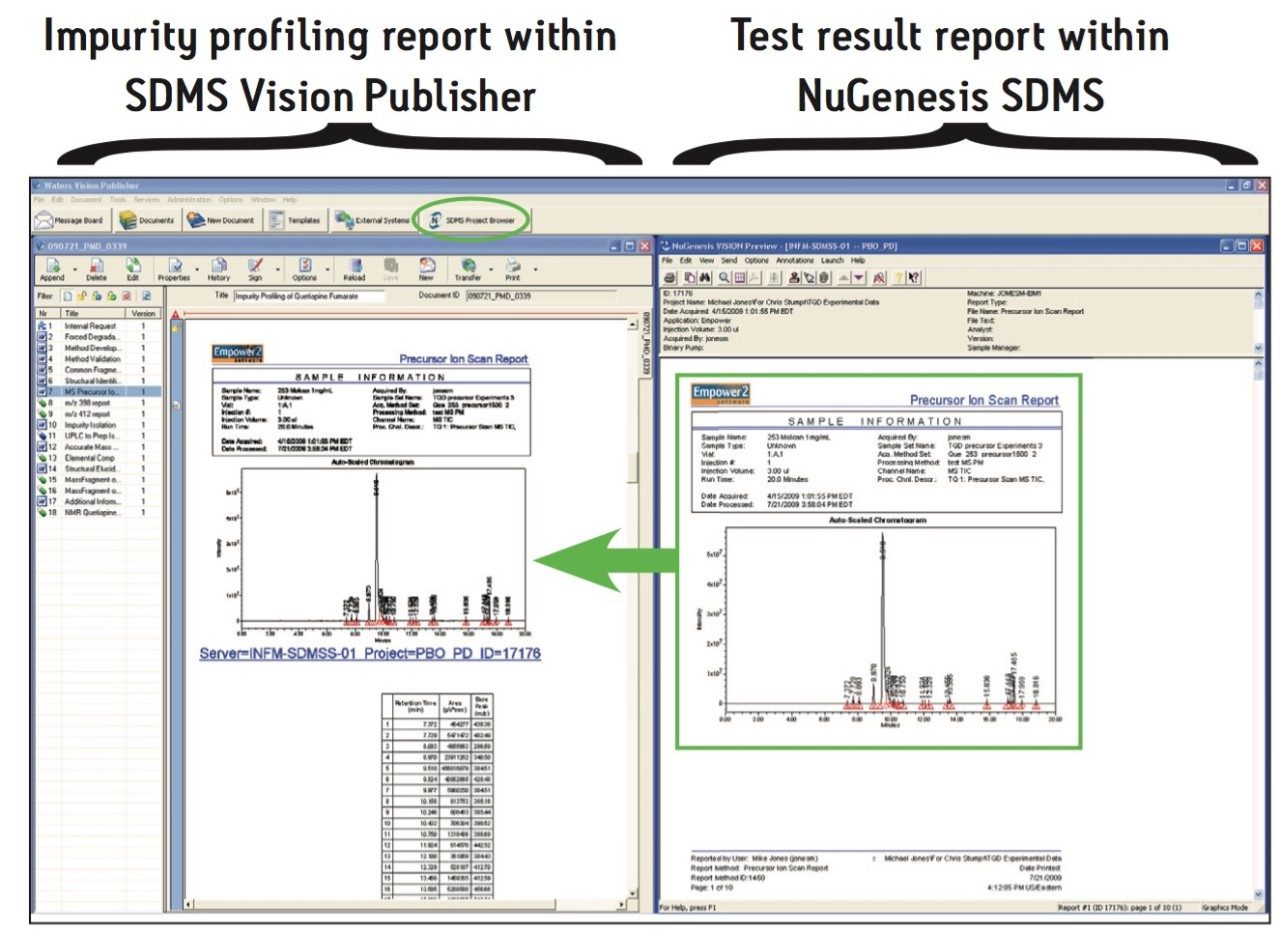
The integration of SDMS Vision Publisher with NuGenesis SDMS provides many benefits to the laboratory scientist and their management. For example, the SDMS Vision Publisher report is traceable back to the original results by following hyperlinks. Completed reports are finalized by electronic review and sign-off. In addition, by using SDMS Vision Publisher for report creation along with electronic review and sign-off, the time and effort required to create impurity profiling reports is dramatically reduced thereby enhancing the productivity of scientists and the analytical laboratory.
NuGenesis SDMS and SDMS Vision Publisher streamline data management and report authoring for all analytical laboratory experiments including impurity profiling. Key benefits of this integrated Informatics solution include:
720003704, September 2010