
Focused gradients improve the resolution between closely eluting peaks without increasing chromatographic run time. Improved resolution leads to product with higher purity and increased yield.
Chromatographic separations for isolation and purification are governed by the same physical and chemical principles as analytical separations. In prep experiments, however, scientists isolate compounds at high mass loads, often on large columns, and require better resolution to enhance purity and recovery of the collected materials. Although creating a shallower gradient is a good first approach to enhancing resolution, changing the gradient slope for the whole separation leads to broader peaks and an increase in total run time. Focused gradients, an alternative to universally shallower gradients, decrease the gradient slope for only that portion of the chromatogram that needs increased resolution, providing more resolution between closely eluting peaks without increasing the total run time. A focused gradient can be defined based on a scouting run or directly from a first prep run.
Steps for gradient development:
|
LC system: |
Waters 2525 Binary Gradient Module, 2767 Sample Manager, Column Fluidics Organizer, 2996 Photodiode Array Detector, AutoPurification Flow Cell |
|
Column: |
XBridge Prep OBD C18 Column 19 x 50 mm, 5 μm (Part Number 186002977) |
|
Flow rate: |
25 mL/min |
|
Mobile phase A: |
0.1% Formic acid in water |
|
Mobile phase B: |
0.1% Formic acid in acetonitrile |
|
Wavelength: |
260 nm |
*Peak selected for focused gradient.
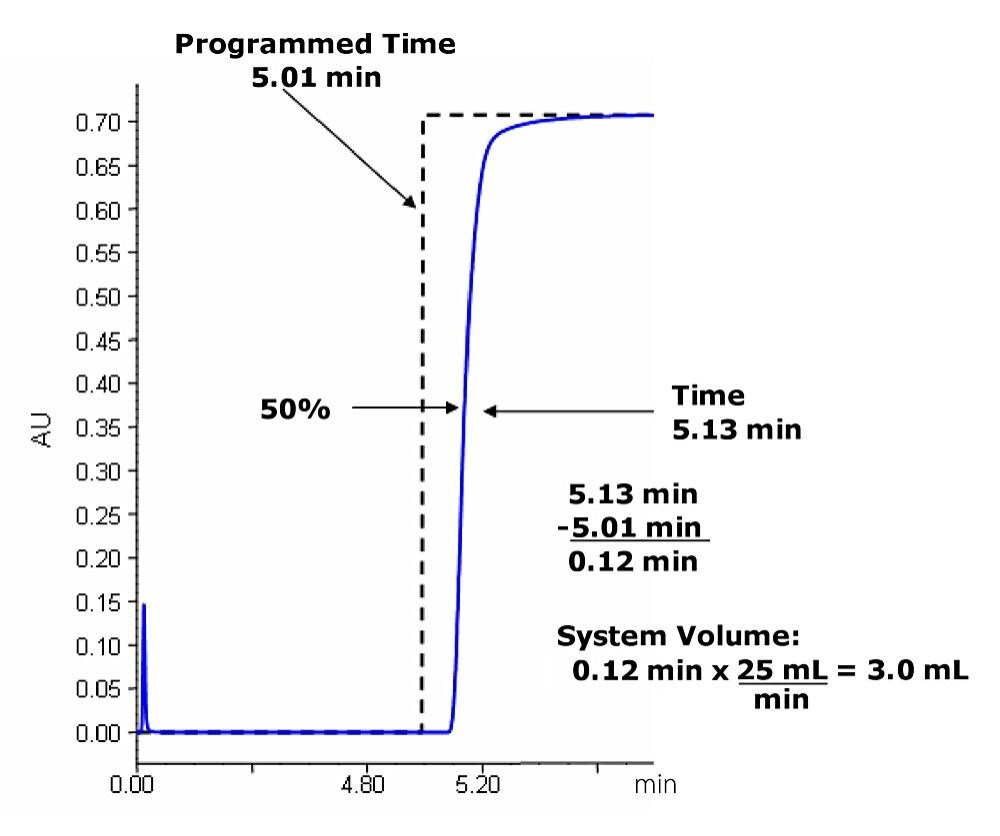
The system volume is defined as the volume from the point of gradient formation to the head of the column. The system volume is used in designing the focused gradient. As shown in Figure 1, the system volume for the instrument configuration used in this experiment is 3.0 mL.
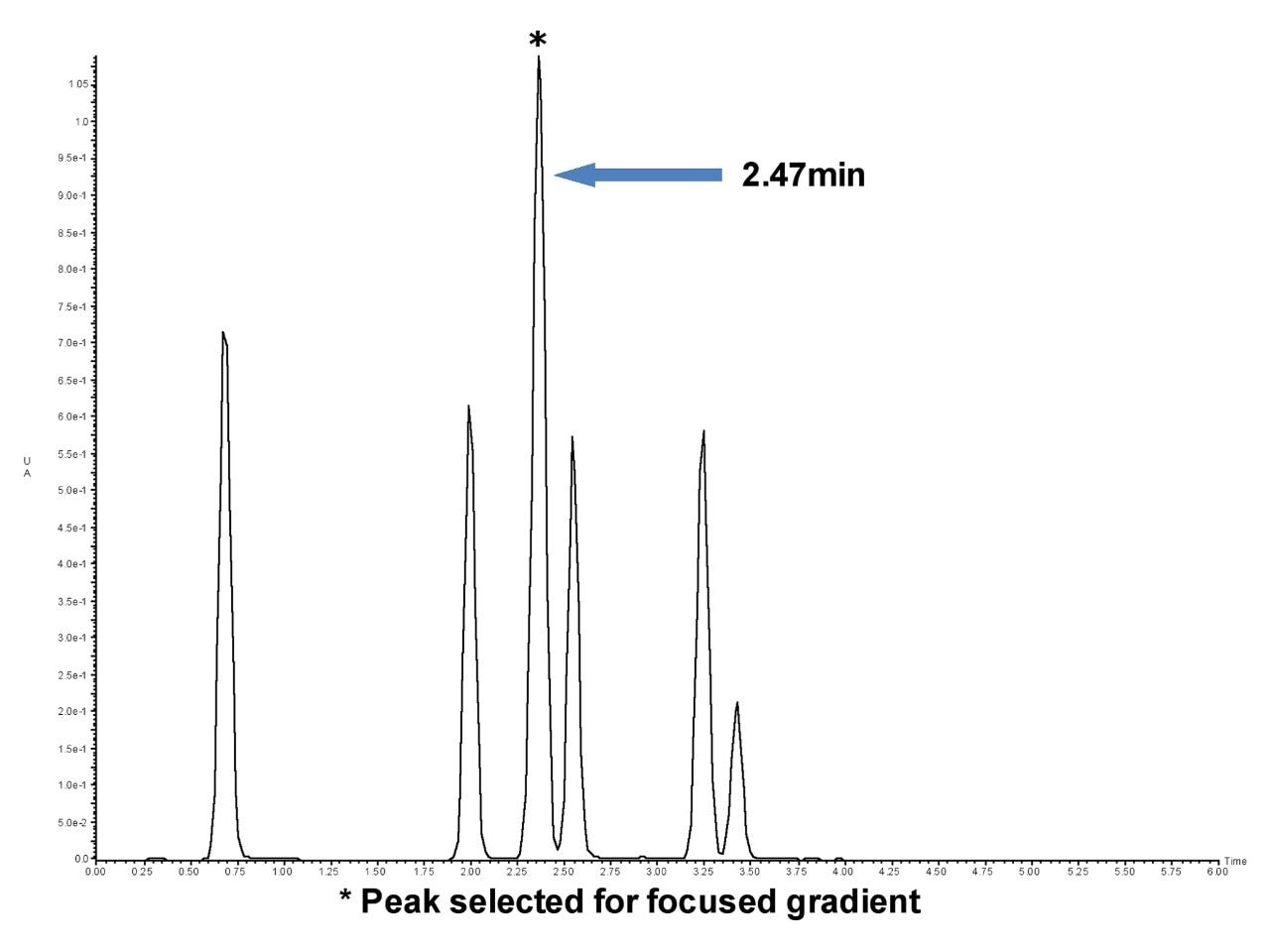
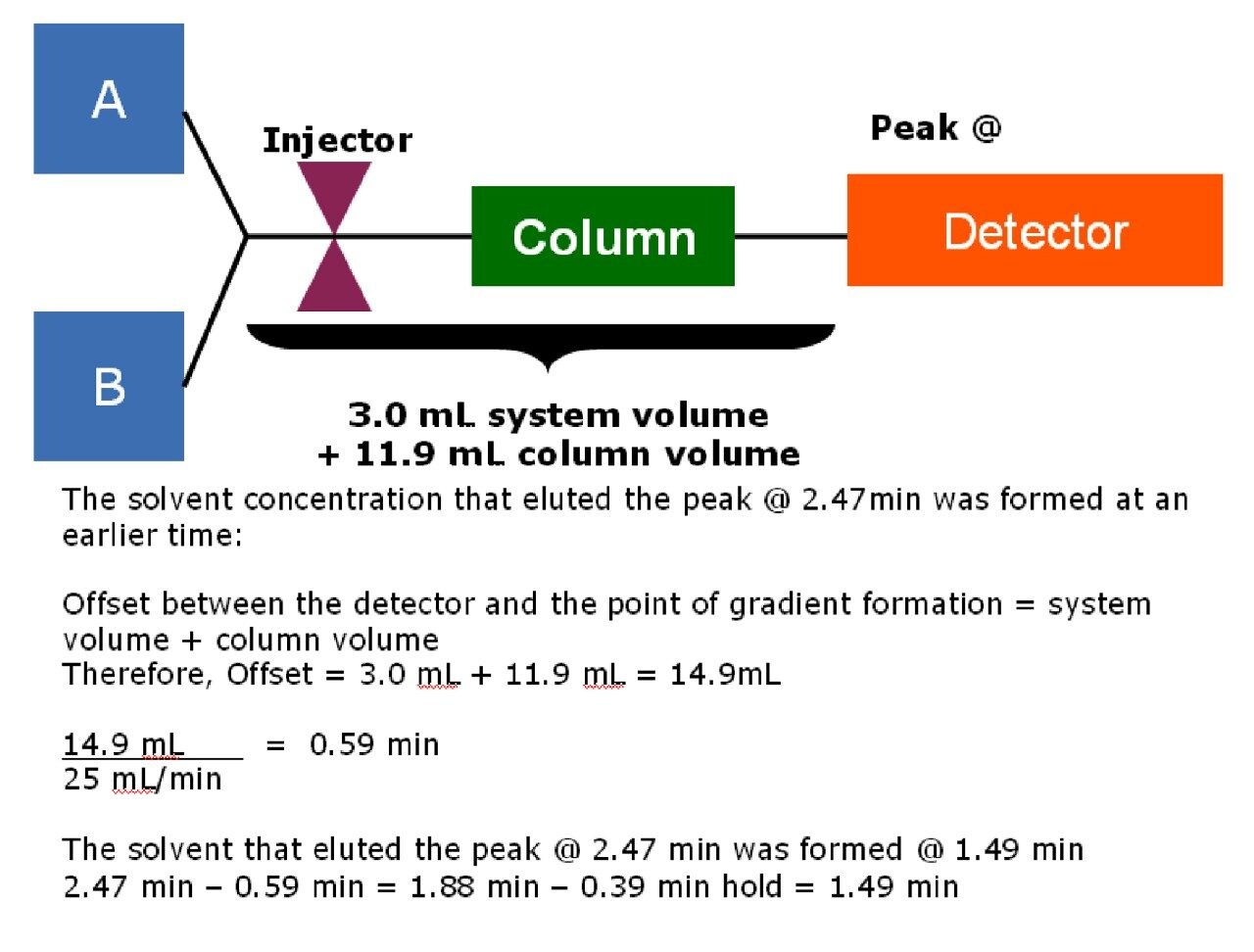
The solvent concentration that elutes peak 3 at 2.47 minutes was formed at an earlier time. As shown in Figure 3, the offset between the detector and the point of gradient formation is equal to the system volume plus the column volume. The offset, then, for this particular system is equal to the 3 mL system volume determined earlier plus the volume of the 19 x 50 mm prep column (11.9 mL), or 14.9 mL. At a flow rate of 25 mL/min, it takes 0.59 minutes for the solvent concentration to reach the detector. The elution time of 2.47 minutes minus the offset time of 0.59 minutes is 1.88 minutes. Since the initial large scale gradient has a hold of 0.39 minutes, the time when the percentage of acetonitrile that elutes the peak was formed at 1.88 minutes minus 0.39 minutes, or 1.49 minutes into the 5 minute gradient.
Calculate the percentage of acetonitrile that elutes the peak at 2.47 minutes. The original large scale gradient goes from 5-50% B in 5 minutes with an initial hold of 0.39 minutes.
45%
5 min = 9% per min
AND
9% x 1.49 min = 13.4% acetonitrile
min
Another way to calculate the percentage of acetonitrile:
1.49 min x 45% = 13.4% acetonitrile
5.00 min
The percentage of acetonitrile calculated from the gradient that elutes the peak at 2.47 minutes is 13.4%; because the gradient starts at 5% acetonitrile, the actual concentration of acetonitrile that elutes the peak is 13.4% + 5%, or 18.4% acetonitrile.
A focused gradient intended to separate closely eluting peaks in the middle of the gradient should start at the original small pilot scale conditions, usually 0-5% B. Once the sample injection occurs, quickly ramp the gradient to the percentage of acetonitrile that is 5% below the expected percent acetonitrile concentration that will elute the peak of interest. Make the shallow, focused portion of the gradient proceed at one-fifth of the slope that was used in the scouting gradient. A five-fold shallower gradient can be expected to give better resolution between closely eluting peaks. End the focused portion of the gradient 5% above the expected percent acetonitrile concentration that will elute the peak of interest. The original gradient goes from 5-50% B in 5 minutes, or 45% change in 5 minutes. This is a 9% change per minute in acetonitrile concentration (round the 9% to 10% to simplify). The new gradient slope should then be one-fifth of 10%, or 2% change per minute. A 2% change per minute for a 10% change in acetonitrile concentration means that the focused gradient time segment for separating peaks 3 and 4 should have a duration of 5 minutes. Once the focused portion of the gradient is completed, quickly ramp the percentage of acetonitrile to 95% B to wash the column. End the gradient at initial conditions after equilibrating the column.
5-45% B = 9% per min (rounded to 10% per min)
2% per minute gradient slope
10% acetonitrile x 1 min x/2% = 5 min
New focused gradient for isolating peak at 2.47 min:
|
Time |
Flow |
%A |
%B |
|---|---|---|---|
|
0 |
25 |
95 |
5 |
|
1 |
25 |
86.6 |
13.4 |
|
6 |
25 |
76.6 |
23.4 |
|
7 |
25 |
5 |
95 |
|
7.4 |
25 |
5 |
95 |
|
7.5 |
25 |
95 |
5 |
|
10.5 |
25 |
95 |
5 |
|
Time |
Flow |
%A |
%B |
|---|---|---|---|
|
0 |
25 |
95 |
5 |
|
0.39 |
25 |
95 |
5 |
|
5.39 |
25 |
50 |
50 |
|
5.89 |
25 |
5 |
95 |
|
6.89 |
25 |
5 |
95 |
|
7.39 |
25 |
95 |
5 |
|
10.5 |
25 |
95 |
5 |
|
Time |
Flow |
%A |
%B |
|---|---|---|---|
|
0 |
25 |
95 |
5 |
|
1 |
25 |
86.6 |
13.4 |
|
6 |
25 |
76.6 |
23.4 |
|
7 |
25 |
5 |
95 |
|
7.4 |
25 |
5 |
95 |
|
7.4 |
25 |
95 |
5 |
|
10.5 |
25 |
95 |
5 |
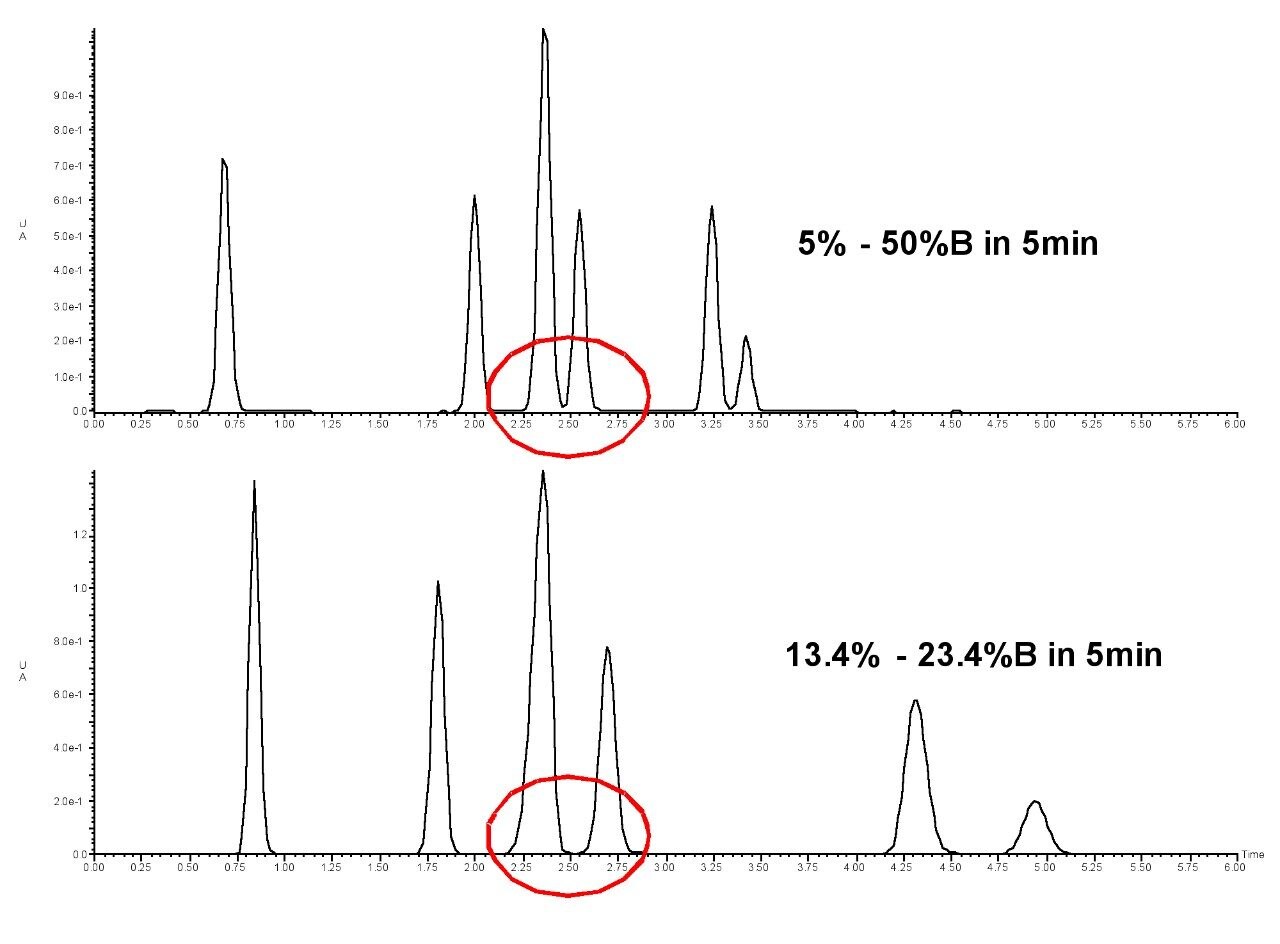
The focused gradient clearly improves the separation between peaks 3 and 4 in the chromatogram in Figure 4. Peaks 5 and 6 shift because they are influenced by the focused portion of the gradient, which continues to elute compounds at the shallower slope until the higher percentage of acetonitrile programmed for column washing permeates the column. Shallow, focused gradients allow the chromatographer to obtain pure products and better recoveries due to better resolution of crude mixture components without an increase in run time.
Scientists isolate compounds at high mass load when purifying products for future experiments. Focused gradients can improve an isolation by refining the resolution between closely eluting peaks without increasing the run time. Knowledge of the system volume permits the direct optimization of the prep gradient. Using focused gradients can increase the product yield and purity without increasing solvent consumption and waste generation. The focused gradient approach to developing isolations, therefore, helps to control purification costs.
720002955, February 2009