This application note demonstrates a fast, robust, and sensitive UPLC-MS method developed for the analysis of synthetic phosphorothioate (PS) oligonucleotides.
Phosphorothioate (PS) oligonucleotides belong to a class of therapeutic agents intended for various indications including cancer and HIV treatment. As therapeutic candidates, PS oligonucleotides must be purified and the remaining minor impurities characterized. The most common contaminants are shorter failure products of synthesis.
Chromatographic resolution of PS oligonucleotides can be difficult or impossible to resolve by conventional ion-pairing reversed-phase methods. These nucleic acid-based therapeutics represent a new class of biopharmaceutical compounds. Analysis of PS oligonucleotides is becoming more important with the revival of antisense and RNAi-based drugs.
The unique LC-MS method presented in this application note resolves the undesirable failed sequences from the target oligonucleotide peak, and characterizes their respective masses.
Previously-described methods employing Waters UltraPerformance LC (UPLC) Technology for the analysis of phosphorothioate oligonucleotides were applied,1 pairing the ACQUITY UPLC System and Oligonucleotide Separation Technology (OST) Columns with MS detection using the Q-Tof Premier Mass Spectrometer.
The exceptional resolution and sensitivity provided by UPLC analysis, used in combination with the high mass accuracy of the Q-Tof Premier, provided an identification of failed phosphorothioate impurities within a 15-minute analysis. Sample throughput has been significantly improved in comparison to HPLC’s 60-minute analysis time.
Mixtures of the phosphorothioate oligonucleotides were analyzed to demonstrate the performance of ACQUITY UPLC System, OST Columns (PN 186003949), and Q-Tof Premier Mass Spectrometer.
Phosphorothioate samples consisting of 25 nucleotides (nt), 5’ –CTC TCG CAC CCA TCT CTC TCC TTC T -3’, and its 24-, 23-, and 22-mer metabolites truncated from the 3’ end were purchased from Integrated DNA Technologies (Coralville, IA).
The samples were reconstituted in mobile phase A to a final concentration of 1 mg/mL. Solvent A consisted of an aqueous solution of 15 mM triethylamine (TEA) containing 400 mM hexafluoroisopropanol (HFIP), pH 7.9. Solvent B contained 50% methanol and 50% solvent A (v/v). Water was used as weak and strong wash solvent. Oligonucleotides of different sizes were premixed in a vial at approximately equimolar ratios.
Introduction of phosphorothioate moieties in the oligonucleotide phosphate backbone creates multiple diastereomers. The number of isomers can be calculated as 2n, where “n” represents the number of nucleotide linkages. The isomers are often partially resolved chromatographically, which results in wider peaks than expected.
Some mobile phases tend to suppress the diastereomeric resolution, and are therefore more suitable for analysis of PS oligonucleotides. Ion-pairing aqueous buffers composed of TEA and HFIP are recommended.1 In addition, the TEA/HFIP based mobile phases are more appropriate for LC-MS and do not cause ion suppression.
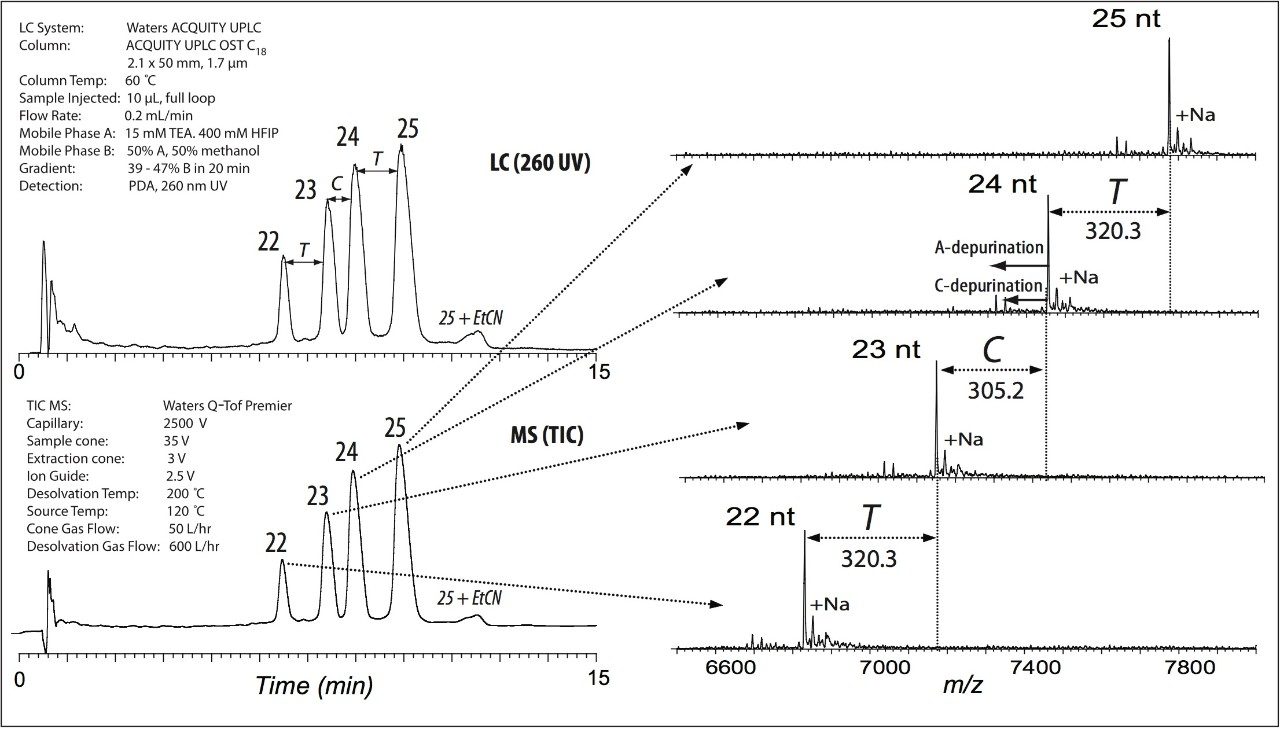
To demonstrate a separation of target PS oligonucleotide from its shorter length fragments, we prepared a mixture of the 25 nt and (N-x) homologs, mimicking 3’ exonuclease digestion, because 3’ digestion is the primary in vivo degradation mechanism. Baseline UPLC separation of the target phosphorothioate product from its (N-x) failure sequences was achieved within 15 minutes (Figure 1). To obtain the best elution profile, the oligonucleotide sample should be prepared in the solvent similar to the initial gradient of mobile phase.
Detection was performed using a photodiode array detector, which was connected to the MS using 75 μm I.D. x 80 cm silica capillary tubing. The narrow I.D. capillary was chosen to minimize the post-column peak broadening prior to MS detection.2
MS analysis was performed using parameters optimized for the most efficient electrospray ionization of the oligonucleotides in the negative ion mode.3 The MS chromatogram demonstrated efficient separation of the peaks corresponding to phosphorothioate failed sequences (Figure 1). Another type of impurity, (N+x), that was also resolved from the targeted sample, was a 25 nt carrying an unremoved cyanoethyl protection group used during oligonucleotide synthesis.
Deconvolution of MS peaks was performed by Waters MassLynx Software with automated MaxEnt1 data processing. The 3’-truncated oligomers and their depurinated fragments were assigned by their molecular mass (Figure 1).
The high-quality spectra that were generated demonstrates this method’s ability to adequately desalt the phosphorothioates for mass analysis with minimal interference due to mobile phase components. Undesired cyano-ethylated oligonucleotide contaminants were assigned based on mass difference comparisons.
A fast, robust, and sensitive UPLC-MS method was developed for the analysis of synthetic phosphorothioate (PS) oligonucleotides. Failed sequences were resolved from the target 25 nt within 15 minutes. The chosen mobile phase allows for an efficient and robust LC-MS analysis of therapeutic PS oligonucleotides.
To the best of our knowledge, no other method offers the required resolution for the analysis of PS class of oligonucleotides. Because of the revival of nucleic acid-based drug research, the biopharmaceutical industry is in critical need for methods for oligonucleotide analysis. The above-described LC-MS method has been successfully adopted in many industrial laboratories around the world.
Among the benefits of the UPLC methodology is its reduced sample analysis time, which improves sample throughput by approximately four-fold compared to traditional HPLC methods for PS oligonucleotide characterization.4
The adoption of LC/UV and LC-MS methods based on Waters UPLC Technology will increase productivity and reduce analysis costs for laboratories involved in the discovery, design, and commercialization of this class of biologically-significant compounds.
720002621, June 2008