Here we present a method that provides efficient desolvation and effective transport of large biological ions from atmospheric pressure into the vacuum system of a mass spectrometer, through modification of the atmospheric pressure gas (cone gas) composition. No other source modifications were carried out in this work. Significant enhancements of the transmission and desolvation of large multiply-charged protein ions were achieved by changing the cone gas from nitrogen to a signficantly heavier polyatomic gas.
Here, we present a method that provides efficient desolvation and effective transport of large biological ions from atmospheric pressure into the vacuum system of a mass spectrometer, through modification of the atmospheric pressure gas (cone gas) composition. No other source modifications were carried out in this work.
Significant enhancements of the transmission and desolvation of large multiply-charged protein ions were achieved by changing the cone gas from nitrogen to a significantly heavier polyatomic gas. Nanoelectrospray is an ionization technique that efficiently generates large biological gas-phase ions. Transfer of non-covalently associated protein-protein complexes from solution to the gas phase generally results in the formation of ions possessing relatively few charges, and consequently m/z values are often above 10,000. This depends on the size of the protein complex under investigation.
Biological samples analyzed under non-denaturing aqueous conditions are heavily solvated, resulting in non-gaussian mass spectral peak shape. An efficient desolvation process results in an accurately measured intact mass of the complex, due to the stripping of non-specific solution adducts.
The instrument used in these studies was a SYNAPT HDMS System, which has a hybrid quadrupole/IMS/oa-ToF geometry. Briefly, samples were introduced by a borosilcate glass nanoelectrospray spray tip and sampled into the vacuum system through a Z-Spray source. The ions passed through a quadrupole mass filter to the IMS section of the instrument. This section is comprised of three travelling wave (T-Wave) ion guides. The trap T-Wave accumulates ions, while the previous mobility separation occurs. These ions were released in a packet into the IMS T-Wave in which the mobility separation was performed. The transfer T-Wave was used to deliver the mobility separated ions into the oa-ToF analyzer.
Alcohol dehydrogenase (147.5 kDa), GroEL-SR (400 kDa, single ring), Proteasome-S (700 kDa), and GroEL (800 kDa) were all buffer exchanged into an aqueous solution of 100 mM ammonium acetate, to a final working protein concentration of 1.5 μM. Sulphur hexafluoride (SF6) was obtained from BOC Gases LTD. Octafluoropropane was obtained from F2-Chemicals, UK.
SF6 and Octafluoropropane were introduced as cone gas through the sheath cone assembly, as shown in Figure 1. The flow rate was controlled by means of a software controlled Bronkhorst gas flow controller (standard). The flow rate was varied from 0 L/hour to 100 L/hour to investigate optimal conditions for GroEL (800 kDa) detection and transmission.
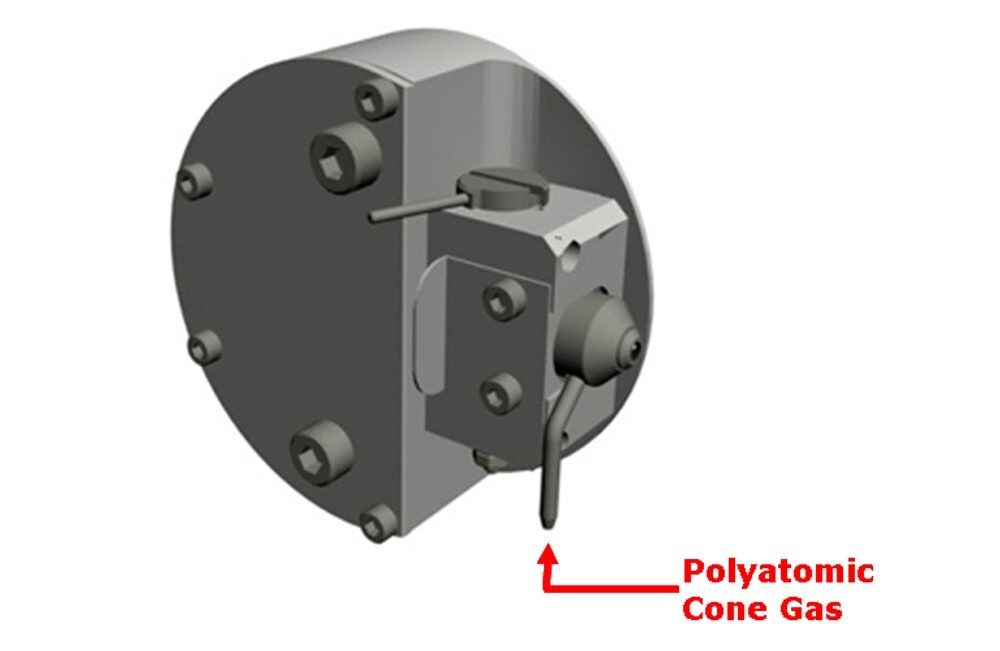
To enable accurate comparisons of GroEL transmission when assessing SF6 and Octafluoropropane, the deconvolution algorithm Maximum Entropy 1 was used to generate an intensity value for the entire GroEL multiply-charged envelope. This figure was then plotted against the cone gas flow rate, as shown in Figure 2. SF6, when used as a cone gas, “cools” the large ions more efficiently than Octafluoropropane. The multiply-charged GroEL ions were not detectable when Nitrogen, Argon, or Xenon (data not shown) was used as a cone gas.
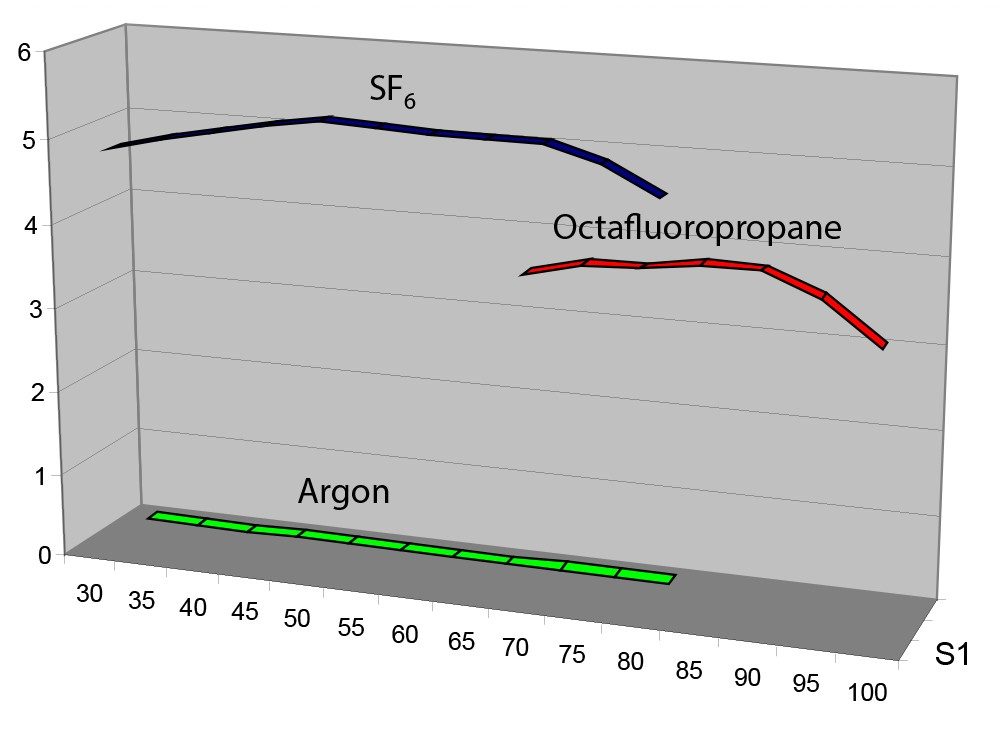
Large multiply-charged ions possess a large energy spread within the mass spectrometer. The kinetic energy spread reduced, so the ions could be focused and ultimately detected. Polyatomic gases, such as SF6 and Octaflouropropane, “cool” or “thermalize” large ions, as shown in Figures 3 and 4, which resulted in enhanced detection. Polyatomic gases possess far more degrees-of-freedom than monoatomic gasses, such as Argon or Xenon. For example, SF6 (Mw 146) and Octafluorpropane (Mr 188) possess 21 and 33 degreesof- freedom (3N) respectively, as opposed to the 3 degrees-of-freedom of Xenon (Mr 131).
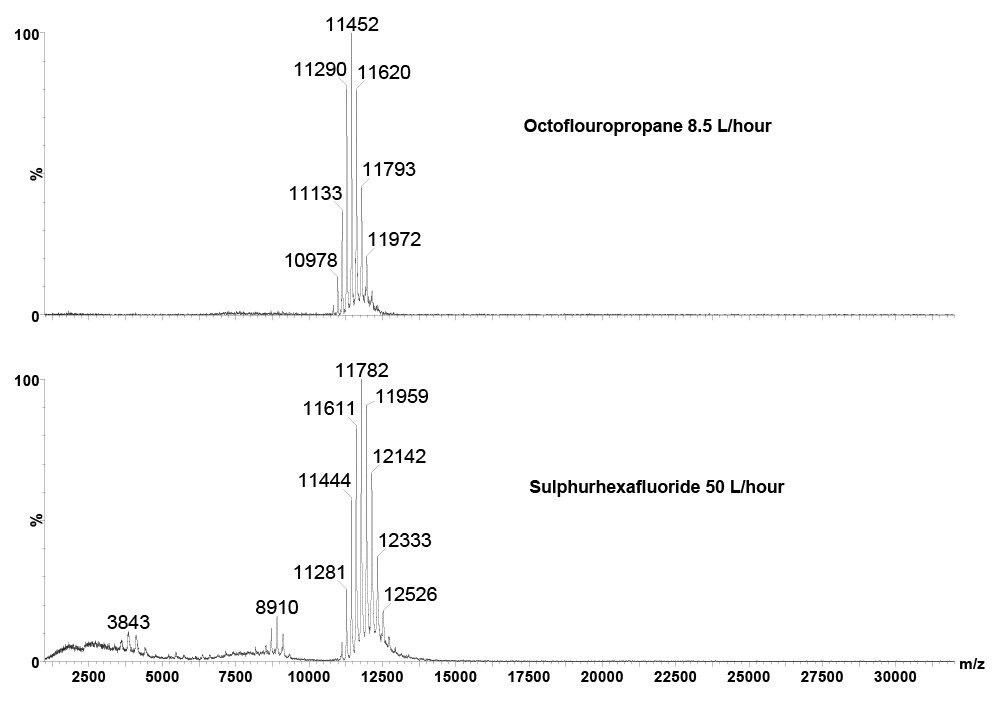
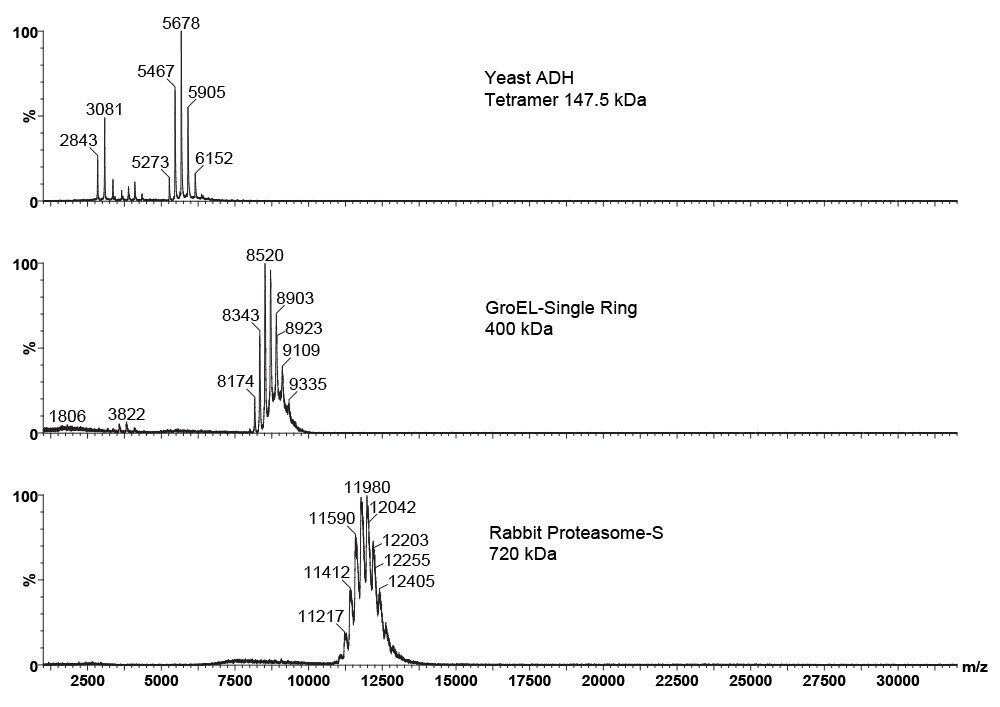
Polyatomic gases used as a cone gas dramatically improved transmission and desolvation of large multiply charged, high m/z ions without the need for any source modification.
Improved transmission and efficient desolvation of large multiply-charged ions allowed for accurate mass measurement of large protein/protein noncovalent complexes.
720002744, July 2008