This is an Application Brief and does not contain a detailed Experimental section.
In this aaplication brief, we investigate and present the use of monoatomic (Xenon and Argon) and polyatomic (Sulphurhexafluoride and Octafluoropropane) gases as collision gases when performing MS/MS fragmentation of large multiply-charged precursor ions.
Provided subunit composition information on biological macromolecules whose subunit stoichiometry may not be known.
The current Waters SYNAPT HDMS Mass Spectrometer and nanoelectrospray sources lend themselves particularly well to preserving non-covalent interactions, allowing one to analyze compounds in their native conformation and stoichiometry1. The transfer of non-covalently associated complexes from solution to the gas phase using electrospray ionization generally results in the formation of ions possessing relatively few charges. Therefore, these species appear high on the m/z scale, which makes Time-of-Flight (ToF) mass spectrometry ideal for their mass analysis.
Utilizing a 32k amu quadrupole allows for selection and fragmentation of these multiply charged species that appear high on the m/z scale. The benefit of this method is twofold: fragmentation of large macromolecular complexes and determination of individual subunit mass and stiochiometry.
Fragmentation of large, highly-charged species enables product ions to possess a wide kinetic energy spread. This energy spread needs to be thermalized. This is achieved by the presence of a collision gas in the Trap T-Wave region of the SYNAPT HDMS System.
Here we investigate and present the use of monoatomic (Xenon and Argon) and polyatomic (Sulphurhexafluoride and Octafluoropropane) gases as collision gases when performing MS/MS fragmentation of large multiply-charged precursor ions. In previous studies Argon has either been mixed with, or replaced with, larger inert monoatomic gases2,3 to improve fragmentation efficiency or transmission of high m/z ions. Polyatomic gases are particularly efficient at thermalizing large ions because they have many degrees of freedom. For example, SF6 (Mw 146) and Octafluorpropane (Mr 188) possess 21 and 33 degrees of freedom (3N) respectively, as opposed to the 3 degrees of freedom of Argon (Mr 40) Xenon (Mr 131).
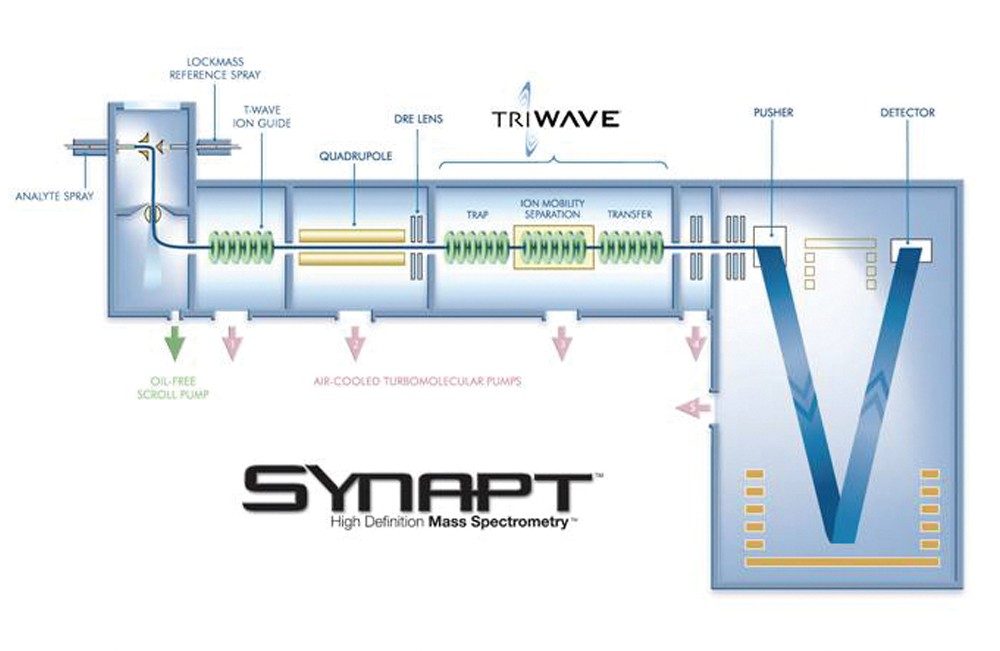
The instrument used in this study was a SYNAPT HDMS System, which combines high-efficiency ion mobility based measurements and separations with a hybrid quadrupole orthogonal acceleration Time-of-Flight (oa-ToF) mass spectrometer, as shown in Figure 1.
Samples were introduced with a borosilcate glass nanoelectrospray-spray tip and sampled into the vacuum system. The ions pass through a quadrupole mass filter to the enabling Triwave device consisting of three travelling wave (T-Wave) ion guides4. The gas pressure in the TRAP and TRANSFER T-Wave regions varied from 2.4 e-2 to 6.7 e-2 mbar, which was dependant on which collision gas was used. The IMS T-Wave was operated at 0.5 mbar (Nitrogen), but not in ion mobility mode.
|
MS System: |
SYNAPT HDMS System |
|
Ionization Mode: |
nanoESI Positive |
|
Capillary Voltage: |
1,000 V |
|
Cone Voltage: |
150 V |
|
Collision energy: |
50 – 180 V |
|
Acquisition Range: |
1,000 – 50,000 m/z |
|
T-wave Trap/Trans: |
Argon, Xenon, SF6, and gases Octafluoropropane |
|
T-wave Trap/Trans: |
2.4 e-2 to 6.7 e-2 mbar pressures |
Upon infusion of GroEL in aqueous solution into the mass spectrometer, a narrow, well-defined multiply-charged envelope was observed at m/z 12,000, corresponding to intact GroEL mass 801 kDa, as shown in Figure 2.
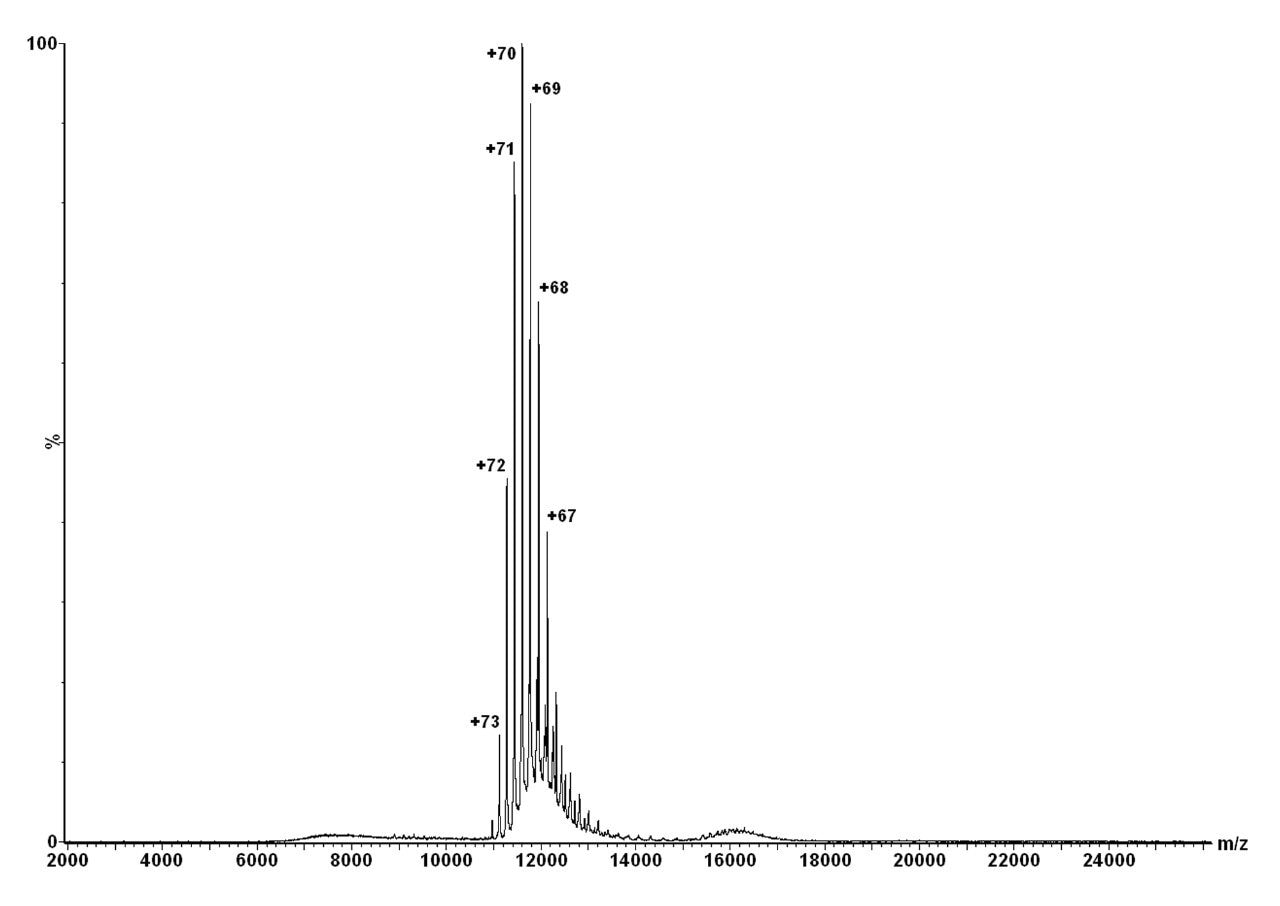
The multiply charged ion m/z 11,440 was then selected by the quadrupole and subjected to different collision energies ranging from 50 V to 180 V, in the presence of four different collision gases (Argon, Xenon, SF6, and Octafluoropropane).
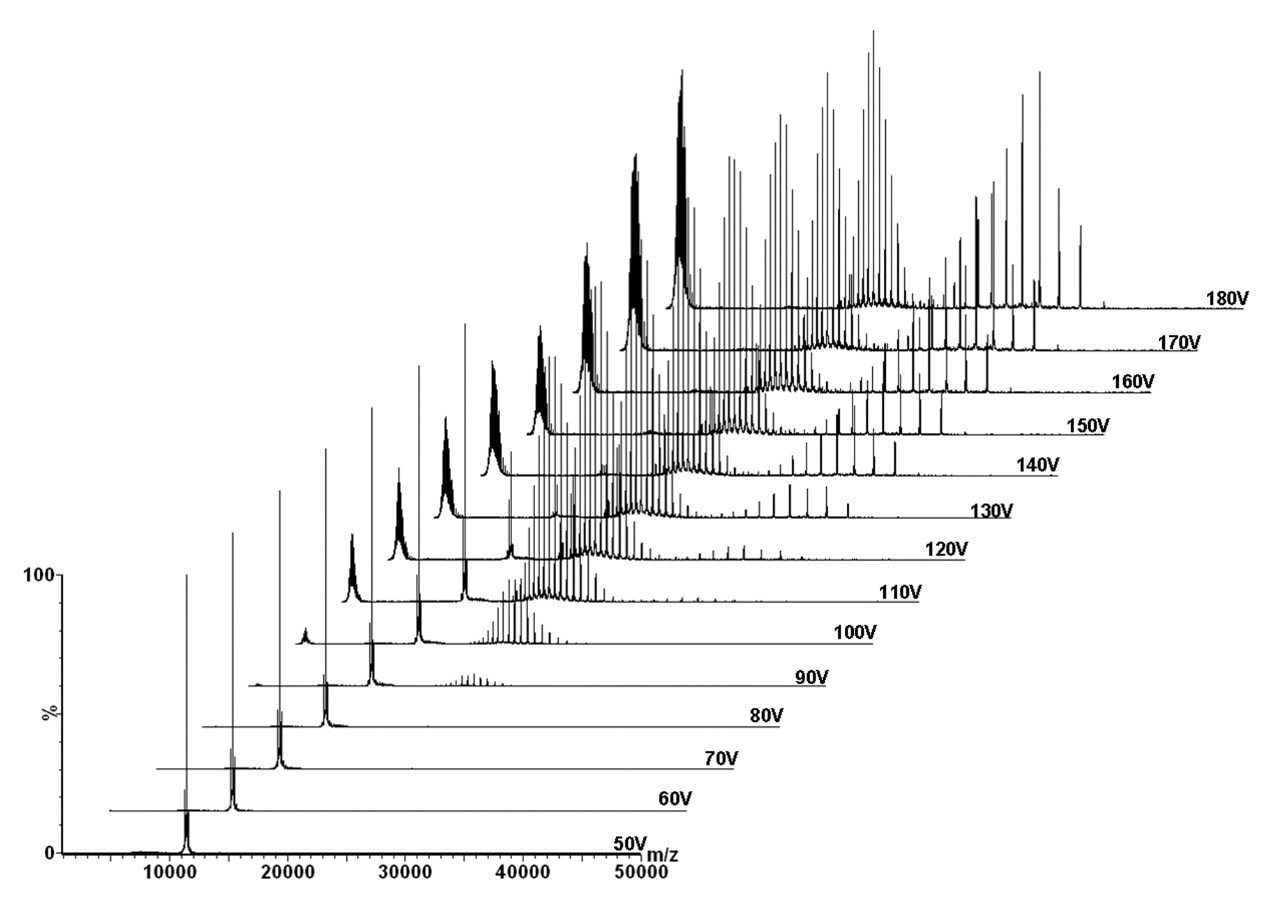
A typical MS/MS fragmentation profile is observed in Figure 3. At low collision energies (50–80 V), the precursor ion GroEL tetradecamer (14 mer) remained intact. At a collision energy of 90 V, the highly charged monomer appeared with the accompanying tridecameric (13 mer) species. At collision energies above 120 V, the appearance of the dodecamer (12 mer) was observed.
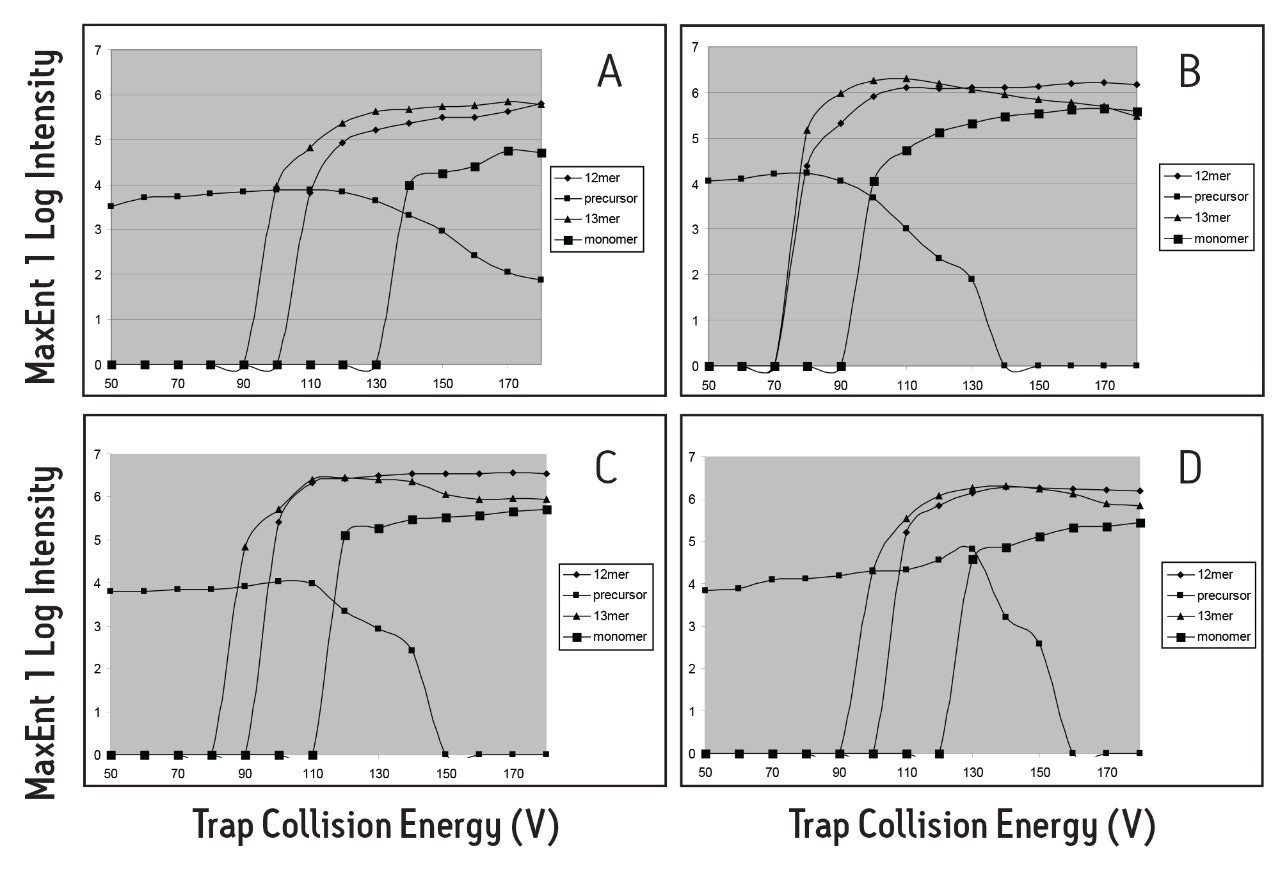
It is observed from Figures 4 A-D that the larger collision gases – Xenon, SF6, and Octafluoropropane – provided improved collisional cooling over smaller gases, such as Argon. SF6 provided improved ion thermalizing properties over Xenon, observed by the increased ion intensities of the Dodecamer (12 mer). The polyatomic gas SF6, with its greater number of degrees of freedom (21), thermalized large ions more efficiently than Xenon or Argon.
High m/z precursor ions were selected in the high mass quadrupole, fragmented in the in T-wave trap region, and subsequently massmeasured in the ToF analyzer, which provided subunit composition information on biological macromolecules whose subunit stoichiometry may not be known.
Polyatomic gases possessed a greater number of degrees of freedom. Therefore, they provided improved collisional cooling of large m/z ions produced by an MS/MS experiment. This resulted in increased sensitivity on high m/z and highly-charged species.
720002743, July 2008