
For research use only. Not for use in diagnostic procedures.
This application note demonstrates the application of UltraPerformance Liquid Chromatography for LC-MS based metabonomics.
Metabonomics has been defined as “The quantitative measurement of time-related multiparametric metabolic responses of multicellular systems to patho-physiological stimuli or genetic modification” [J.K.Nicholson, J.C.Lindon, and J. Everett, Xenobiotica 1999]. Metabonomics studies, as applied to such application areas as target validation, drug efficacy, disease diagnosis, and personalized medicine, are typically performed using biological fluids such as plasma, urine, and CSF. These fluids are studied using information-rich spectroscopic techniques such as proton NMR or mass spectrometry.
The analysis of these complex biological matrices requires a high-resolving separation prior to mass spectroscopic detection in order to minimize ion suppression and maximize sensitivity. The historical method of choice, High Performance Liquid Chromatography (HPLC), typically employs bonded silica stationary phases of particle diameters from 10 μm to 3 μm and instrumental operating back-pressures of up to 6000 psi. Ideally, one must consider a balance of several variables (particle size, column length, flow rate, backpressure, etc.) for the best chromatographic performance. The resolution in such a system is inversely proportional to the square root of the column particle size.
Smaller particles yield lower HETP (height equivalent to a theoretical plate), or higher column efficiency per unit length, as displayed in the flattened van Deemter curves in Figure 1. Thus, when the particle diameter is reduced from 5 μm to 1.7 μm, the resolution increases by a factor of 1.7. Furthermore, as sensitivity is inversely proportional to peak width, peak width is inversely proportional to the square root of efficiency. Reducing the particle size from 5 μm to 1.7 μm results in an increase in sensitivity by a factor of 1.7. Also displayed in Figure 1 is that optimal flow rate is inversely proportional to particle size. Thus, if we again reduce the particle size from 5 μm to 1.7 μm, the optimal flow rate increases by a factor of 3, resulting in a three-fold increase in speed.
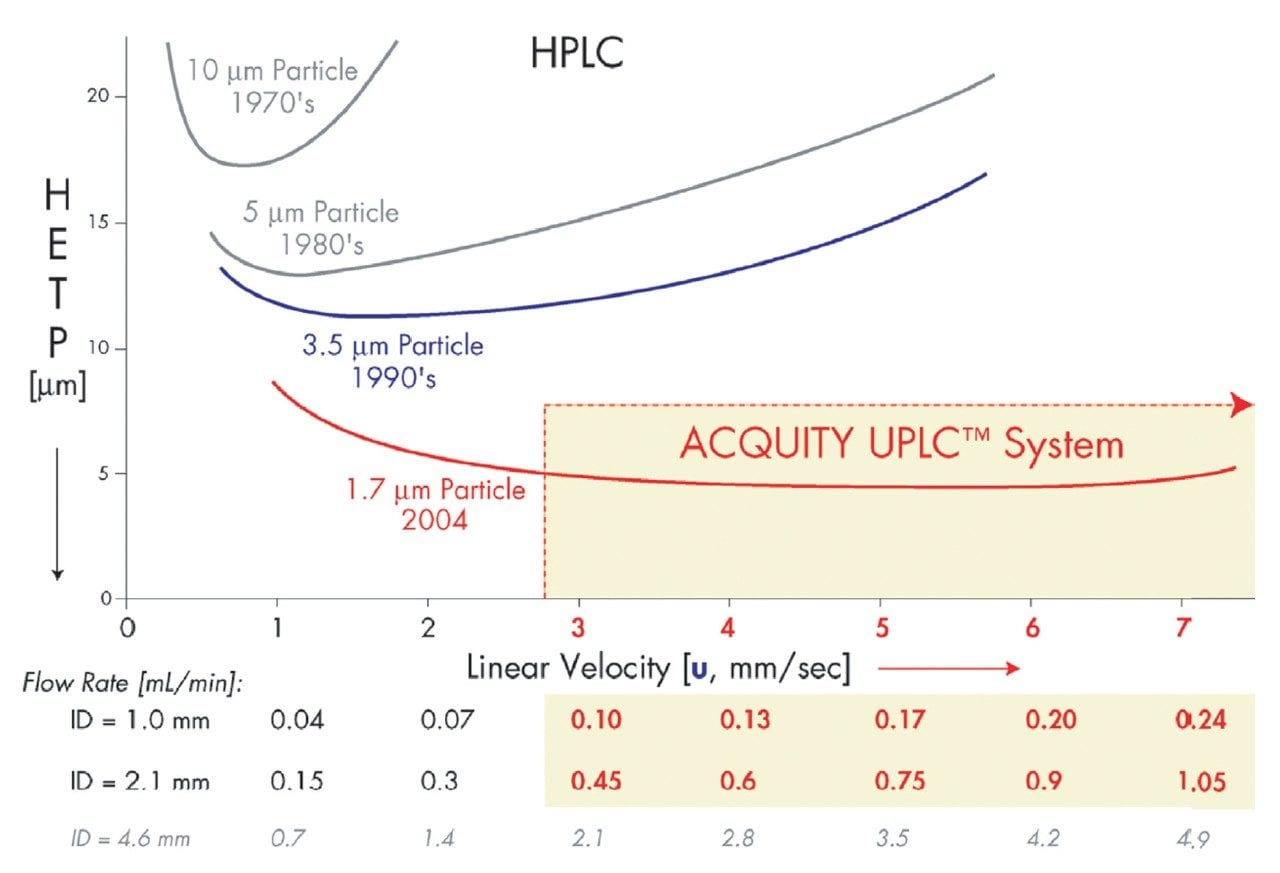
The downside to reducing particle size is the significant increase in column backpressure (inversely proportional to the square of the particle size). Combined with an increase in optimal flow rate, this equates to a 27-fold backpressure increase with a 5 μm to 1.7 μm particle size reduction. In order to harness the potential of sub-2 μm particles without sacrificing performance at optimal flow rates, Waters has developed the ACQUITY UltraPerformance LC System and corresponding ACQUITY UPLC Column chemistries. This system and stationary phase have been holistically designed to withstand these very high pressures (up to 15,000 psi). The ACQUITY UPLC System is an ideal inlet for the Micromass LCT Premier Mass Spectrometer, the high sensitivity orthogonal time-of-flight (oa-Tof) instrument for routine exact mass measurement with a wide dynamic range for high throughput metabonomic screening.
A typical TIC chromatogram produced from an HPLC-MS analysis of a biofluid (2.1 mm x 100 mm, 3.5 μm C18 column and a 10 minute gradient: 0-95% acetonitrile at 600 μL/min) is shown in Figure 2. This chromatogram can be contrasted with that produced by a UPLC-MS biofluid separation in Figure 3 (2.1 mm x 100 mm, 1.7 μm C18 column and a 10 minute gradient: 0–95% acetonitrile at 750 μL/min). It is clear that the UPLC separation yields significantly more peaks (~250) compared that of conventional HPLC (~80–90).
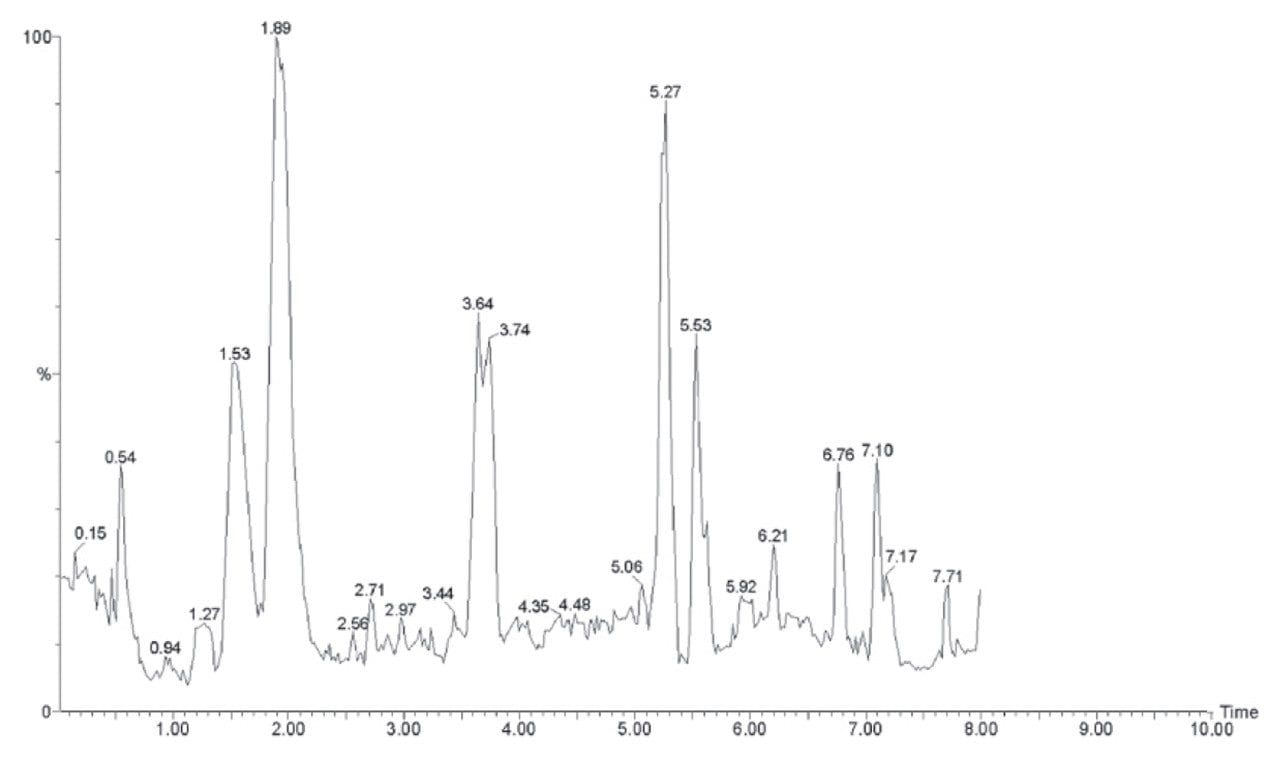
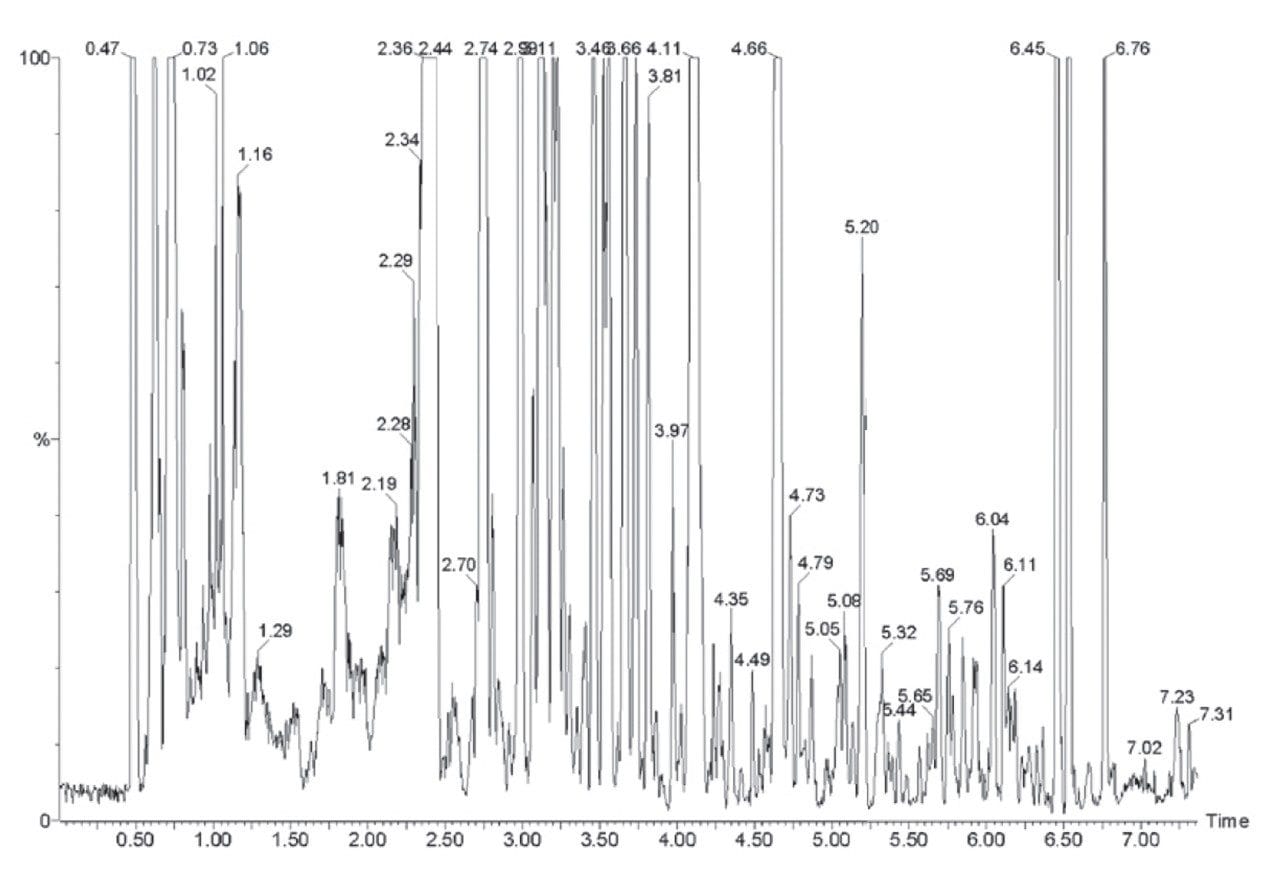
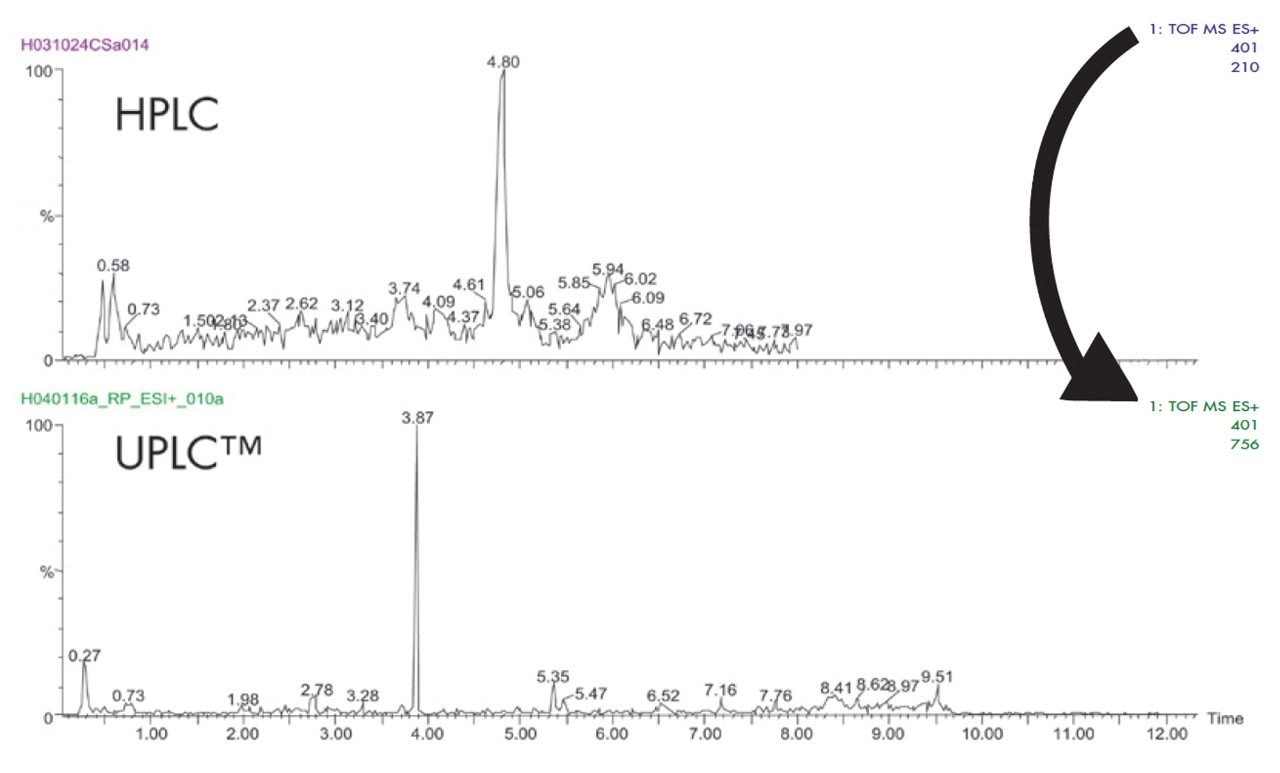
The narrow peak widths generated by UPLC not only contribute to very high peak capacities, but also to an increase in signal response (Figure 4). Chromatographic theory predicts that when moving from 3.5 to a 1.7 μm particle diameter, the signal response should increase by a factor of 1.4. However, when coupled with mass spectrometry detection, we see a three- to five-fold increase in signal response. This increase in sensitivity is a result of the increased resolution and reduction in ion suppression caused by analyte co-elution. The resulting narrow peaks demand a high data acquisition rate from the mass spectrometer in order to obtain sufficient data points across the peaks. The LCT Premier has a data acquisition rate of 20 Hertz, yielding 10–20 points across the LC peak.
The extra resolution and sensitivity produced by UPLC has been exploited for the metabonomic analysis of urine from black, white, and immunosuppressed “nude” mice. The urine samples were collected from both male and female mice (n = 10) in the morning (AM) and in the evening (PM), giving a total of 120 samples. The samples were stored frozen prior to analysis. After defrosting, the samples were centrifuged, the supernatant removed, and diluted 1:4 with distilled water.
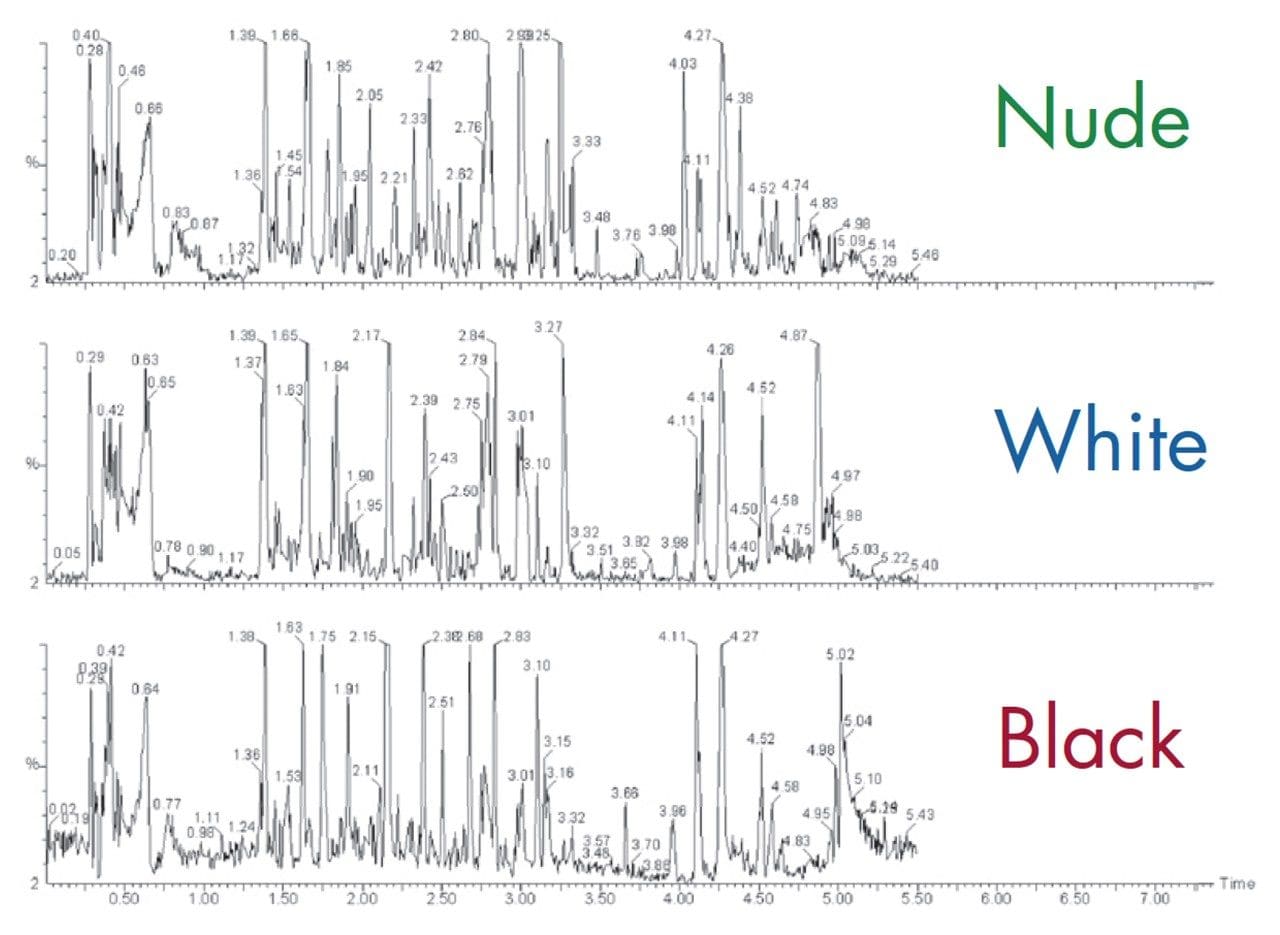
The samples were analyzed by UPLC/oa-Tof MS operating in ESI positive ion mode. A comparison of the 5-minute UPLC-MS chromatogram produced for female AM black, white, and nude mouse urine is shown in Figure 5. We can see that the resulting data is very information-rich, showing subtle differences in the generated chromatograms.
|
LC system: |
ACQUITY UPLC System |
|
Column : |
ACQUITY UPLC Column, C18, 1.7 μm, 2.1 mm x 100 mm |
|
Flow rate: |
750 μL/min |
|
Injection volume: |
5 μL |
|
Gradient: |
Linear gradient of 0–95%, from 0.5–5 min., where A = 0.1% formic acid and B = acetonitrile containing 0.1% formic acid |
|
MS system: |
LCT Premier with LockSpray Ionization Source |
|
Ion mode: |
ESI |
|
Cone voltage: |
60V |
|
Capillary voltage: |
3000V |
|
Desolvation temp.: |
250 °C |
|
Source temp.: |
120 °C |
|
Resolution: |
W-OPTICS 12,000 FWHM |
|
Detection mode: |
Positive ion mode |
|
Dwell time: |
0.05 sec |
|
Collision gas: |
Argon |
|
Lock mass: |
Leucine Enkephalin, 25 fmol/μL |
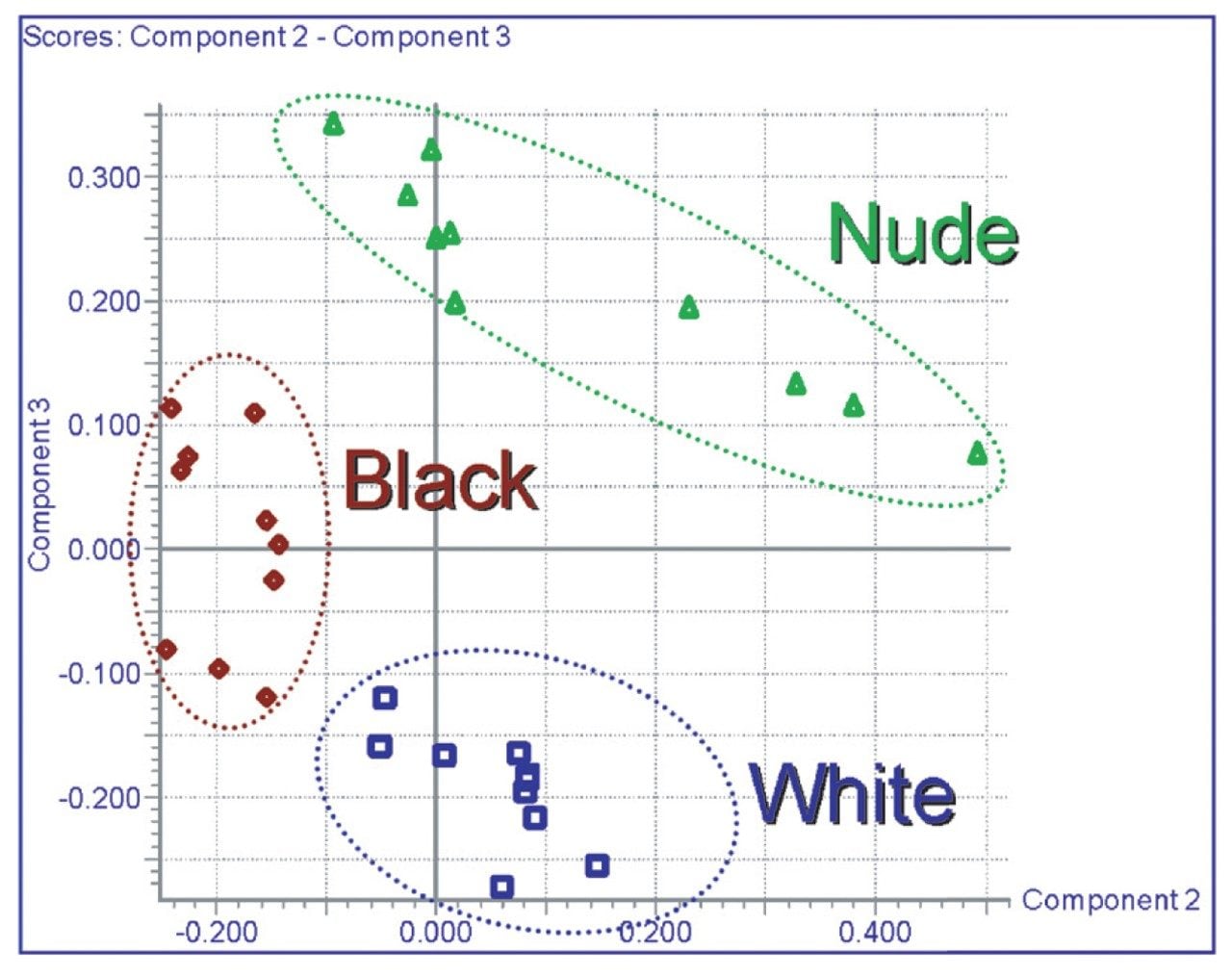
The LC-MS data generated was processed by the MarkerLynx Application Manager for MassLynx v4.0 Software. This generated a total of 9,000 markers in total, some of which were fragment ions. The resulting principal components analysis (PCA) scores plot for the female AM samples is displayed in Figure 6. Here we can see that the three strains of mice are clearly separated into distinct groups, using principal components 2 and 3.
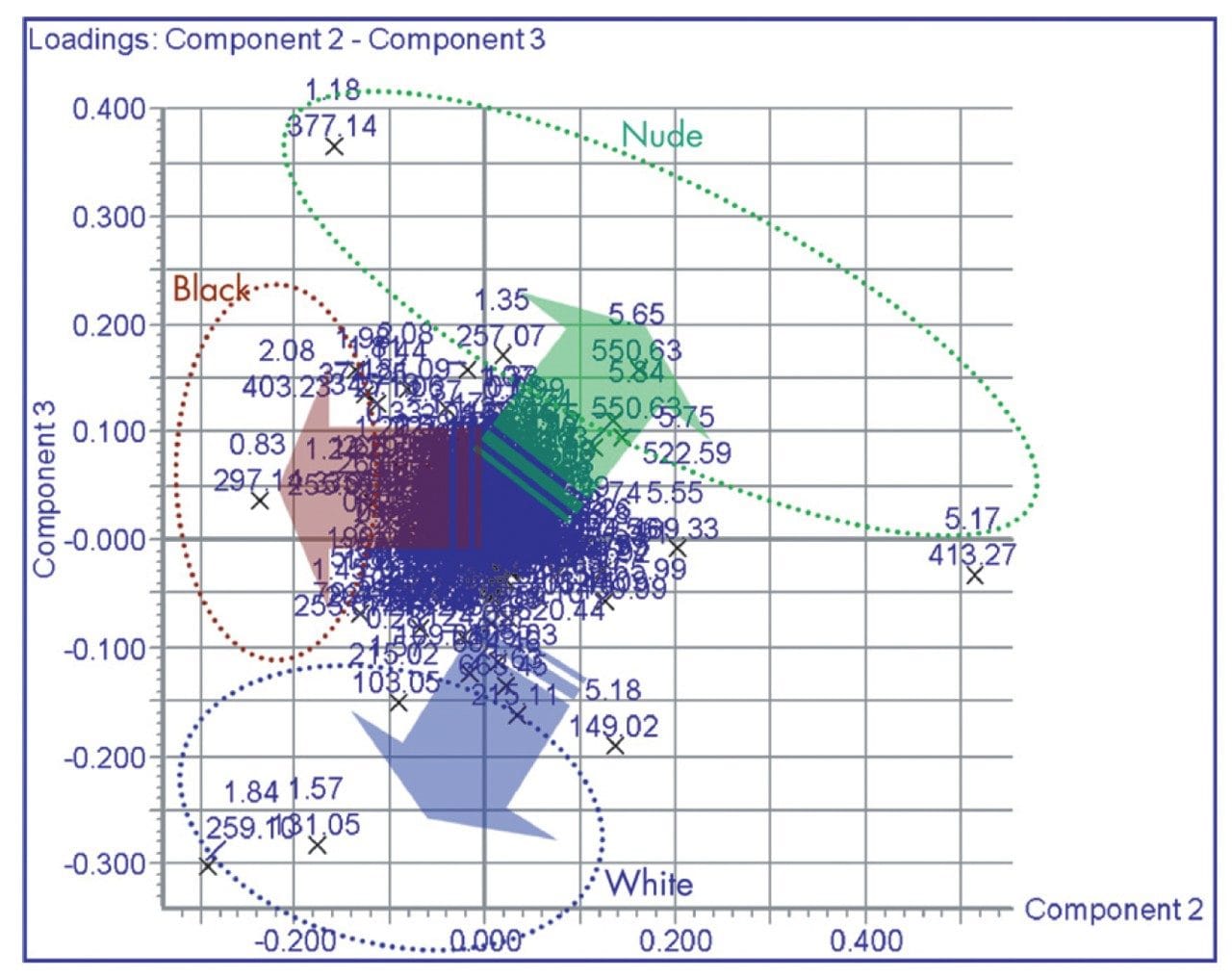
The analytes determining the variance in the data is shown in the loadings plot, Figure 7. Here we can see the retention time and m/z value of the ions of contributing to each of the trajectories of movement for the three groups. From this we can see that the m/z = 259 at 1.8 mins “codes” for the data trajectory for the white mouse urine samples.
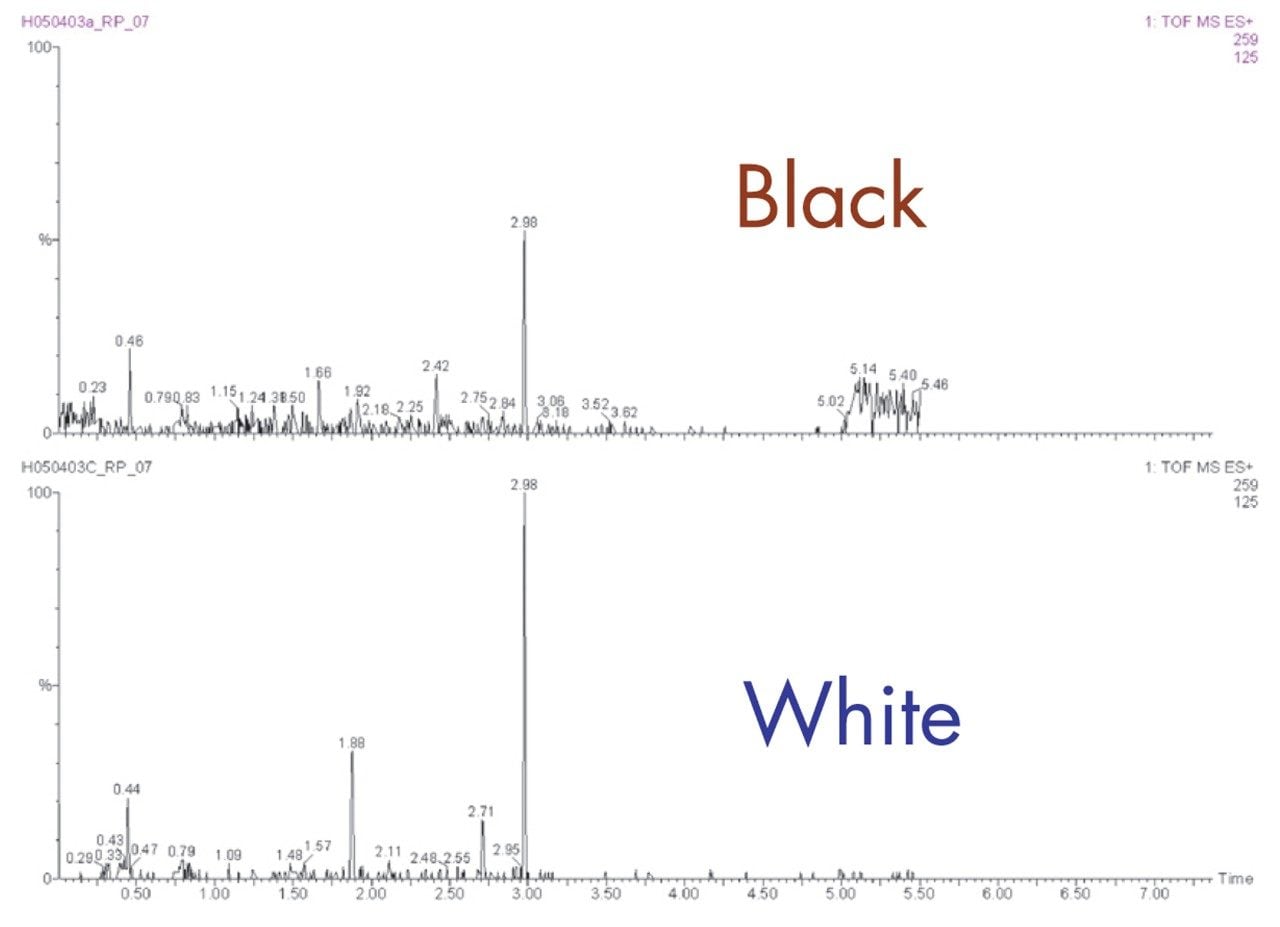
A comparison between the extracted ion m/z=259 for the black and white mouse urine is given in Figure 8. As can be seen from this data, the peak at 1.8 minutes is visible in the white mouse urine but not detected in the black mouse urine. It can also be noted that the peak widths produced are in the order of just 1–2 seconds at the base.
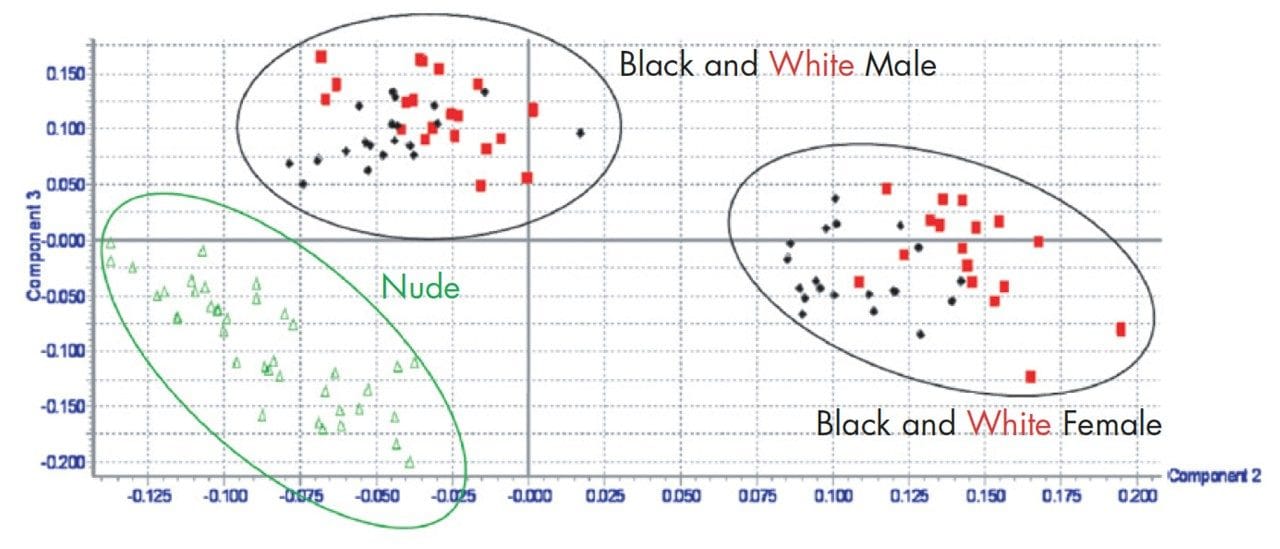
The same mouse urine samples were subjected to re-analysis using a 2.1 mm x 30 mm, 1.7 μm ACQUITY Column and a 0–95% acetonitrile-aqueous formic acid gradient over 1 minute at 800 μL/min. The PCA scores plot generated for AM mouse data is given in Figure 9. This faster analysis generated fewer markers than the 5 minute separation but generated enough data to allow the nude samples to be separated from the black and white samples, with the male and female samples also separated for the black and white mouse samples.
720000866, August 2004