Application Brief
This is an Application Brief and does not contain a detailed Experimental section.
Melissa Aiello1, Kenneth D Berthelette1, Christopher Collins1, Jamie Kalwood1, Thomas H Walter1
1 Waters Corporation, United States
Published on April 25, 2025
This is an Application Brief and does not contain a detailed Experimental section.
This application brief demonstrates increased sensitivity of detection for methylmalonic acid, a small polar acid, using an ACQUITY Premier CSH Phenyl-Hexyl Column equipped with MaxPeak High Performance Surfaces (HPS) Technology, an inert surface hardware. The results were compared to those achieved using a conventional stainless-steel column packed with the same stationary phase.
According to the National Institutes of Health (NIH), approximately 15% of adults aged 51 to 70 have a vitamin B12 deficiency.1 This is especially prevalent in the elderly due to malabsorption of vitamin B12 from food with age. It is also prevalent in populations with food insecurity, and those suffering from pernicious anemia, an autoimmune disorder that harms stomach mucosa, and inhibits proper absorption of nutrients.1–2
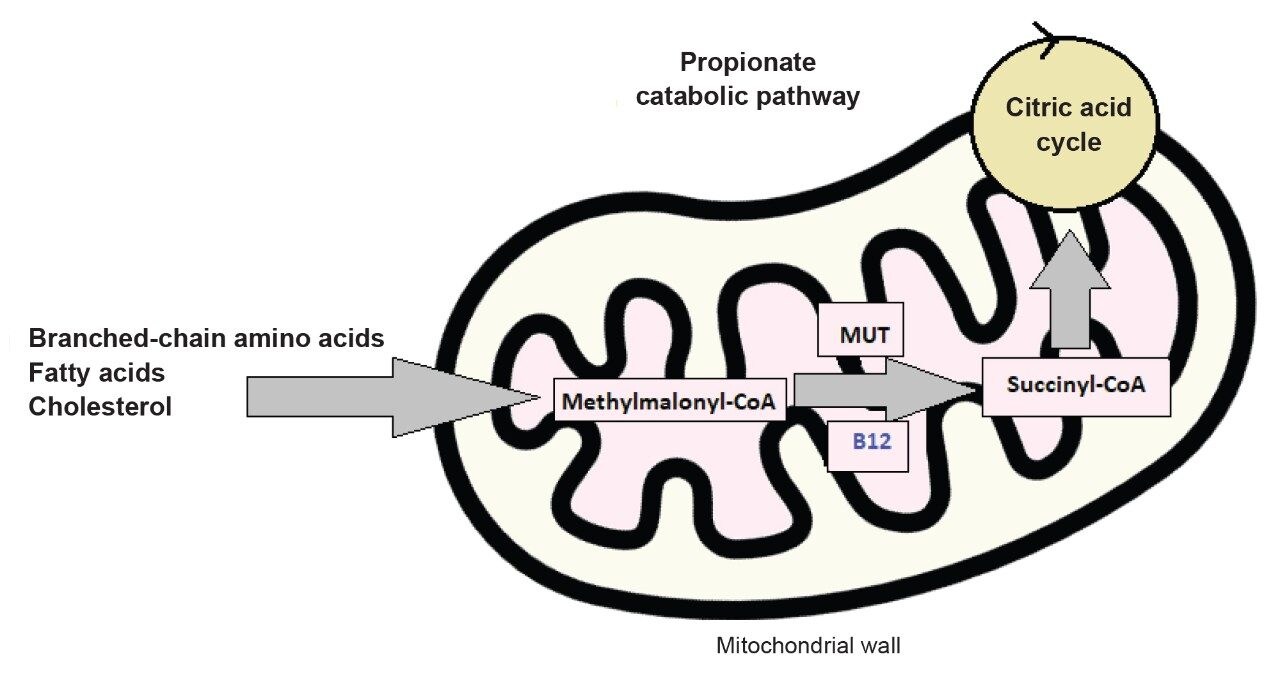
Vitamin B12 is central to many important biologic processes, such as DNA metabolism and red blood cell formation. Therefore, it is crucial to develop accurate clinical tests for determination of serum B12 levels for patients. One way is to measure the amount of methylmalonic acid (MMA) as an indirect determination. MMA is a substrate for the methyl-malonyl-CoA mutase catalyzed conversion of methylmalonyl-CoA to the citric acid cycle-fated succinyl-CoA. This reaction uses B12 as a cofactor and is central to amino acid, fatty acid, and cholesterol catabolism.3,4 Higher levels of MMA indicate that this enzymatic pathway is not functioning properly because there is insufficient B12 to help catalyze the process (Figure 1).
Because methylmalonic acid is a small polar acid, it can be a challenge to analyze using reversed-phase (RP) liquid chromatography. Due to the hydrophobic nature of the sorbent in RP columns, small polar molecules tend to be poorly retained. The use of an appropriate buffer or acid in the mobile phase can keep the polar molecule in its un-ionized form, which will help it interact with the column stationary phase/sorbent. To further aid in the retention of methylmalonic acid, this study used a column with charged-surface hybrid (CSH) particle technology, which uses the BEH (ethylene-bridged-hybrid) particle, which is known to be usable over a wide pH range. The CSH surface modification imparts an overall positive surface charge in acidic mobile phases.5 This positive charge, in conjunction with the phenyl-hexyl ligand, allows the CSH Phenyl-Hexyl column to achieve mixed-mode reversed-phase/anion-exchange separations, which results in increased retention for polar acids.6
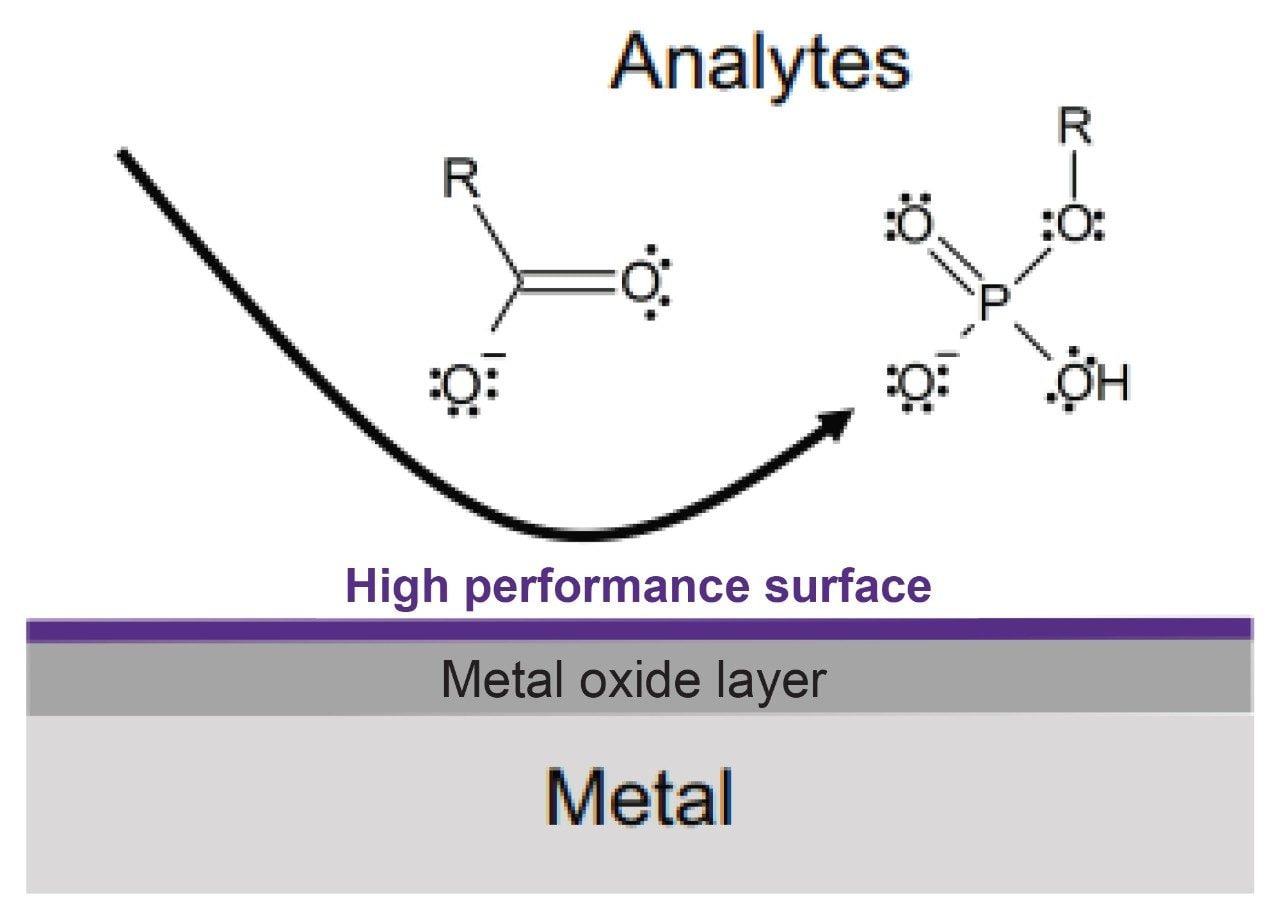
Another analytical challenge is that MMA contains two carboxyl groups, an electron rich group that along with phosphate moieties have been shown to adsorb on the metal surfaces of the chromatographic column and system.6 These interactions can greatly impact analyte recovery, sensitivity, and peak shape. To mitigate these unwanted interactions, MaxPeak Premier Columns employ a high-performance, inert surface applied to the metal components. Specifically, the inert surface consists of a crosslinked layer of ethylene-bridged siloxane groups that provides a barrier against the interaction with acidic probes (Figure 2).7 An additional benefit of this technology is a reduction in the formation of metal ion adducts observed when using electrospray ionization mass spectrometry for detection.6–8
The purpose of this study was to improve the sensitivity in the analysis of methylmalonic acid, a key indicator in clinical vitamin B12 determination. The chromatography obtained using a MaxPeak Premier Column employing inert surface hardware is compared to that of the stainless-steel column counterpart. The impact of the inert surface on mitigating adsorption is investigated, such as increases in peak area and peak height.
Method Conditions
|
LC system: |
ACQUITY I-Class System and Xevo TQ-S micro detector |
|
Mobile Phase A: |
0.1% (v/v) Formic acid in water |
|
Washes/Diluent: |
50:50 (v/v) Methanol and water |
|
Columns: |
ACQUITY Premier CSH Phenyl-Hexyl Column 1.7 µm, 2.1 x 50 mm (p/n: 186009474) ACQUITY CSH Phenyl-Hexyl Column, 130 Å, 1.7 µm, 2.1 mm X 50 mm (p/n: 186005406) |
|
Column temperature: |
30 °C |
|
Sample temperature: |
5 °C |
|
Gradient: |
Isocratic, 100% Mobile Phase A for 2.5 minutes |
|
Flow rate: |
0.555 mL/min |
|
Capillary voltage: |
1.50 kV |
|
Cone voltage, flow: |
10 V, 10 Liters/hour |
|
Desolvation temperature, flow: |
350 °C, 650 Liters/hour |
|
Ion energy, Collision energy: |
0.5 V, 3 V |
|
Scan mode: |
ESI negative, SIR of 117 m/z |
|
Injection volume: |
2 µL |
|
Chromatography software: |
Mass Lynx V4.1 |
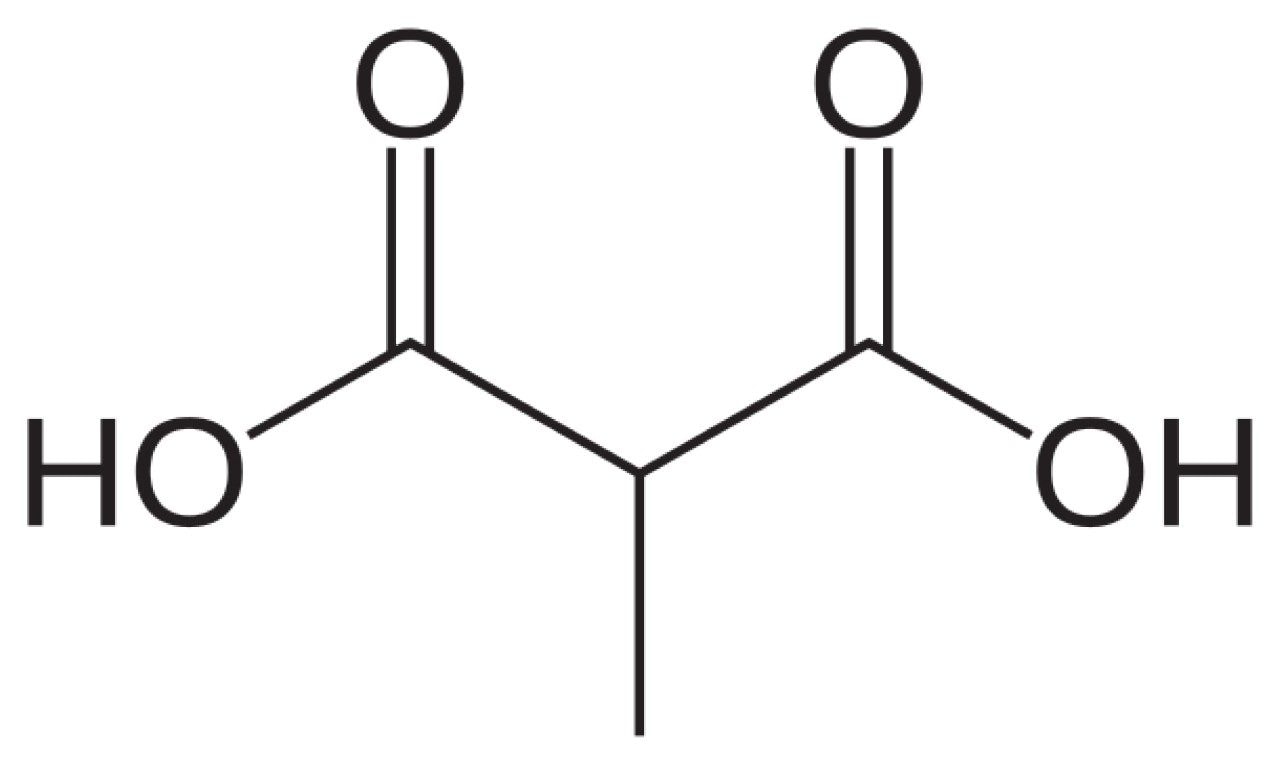
A working standard of methylmalonic acid was prepared in 50:50 (v/v) methanol and water to a final concentration of 0.02 mg/mL. All solutions were stored at 4 ºC, protected from light. The structure of methylmalonic acid is shown in Figure 3. Because methylmalonic acid lacks a chromophore, electrospray ionization mass spectrometry was used for detection.
MMA is a small, polar organic acid and has pKa values of 2.48 and 5.78. Samples were analyzed using an acidic mobile phase (0.1% formic acid in water) with an isocratic method. The columns studied were the ACQUITY Premier CSH Phenyl-Hexyl Column and a stainless-steel version. The CSH stationary phase, which has a positive charge in acidic mobile phases, was used to improve the retention of the partially ionized methylmalonic acid. An overlay of six replicate injections of a MMA standard, for each column type, is shown in Figure 4.
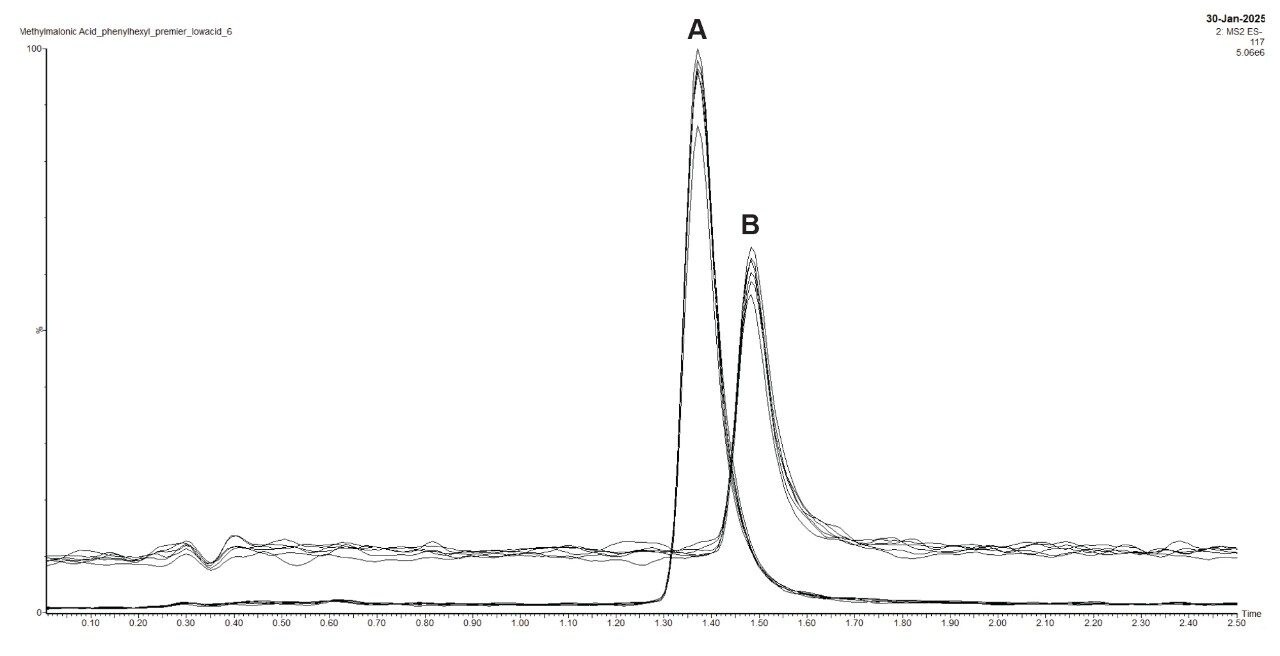
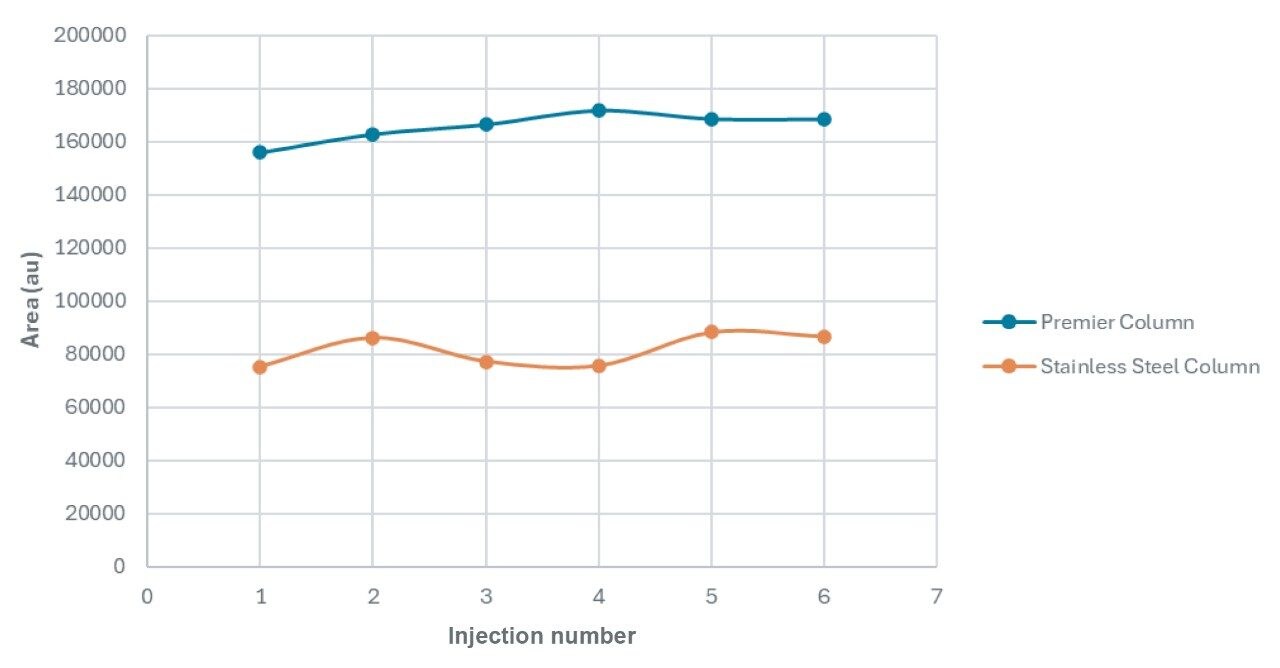
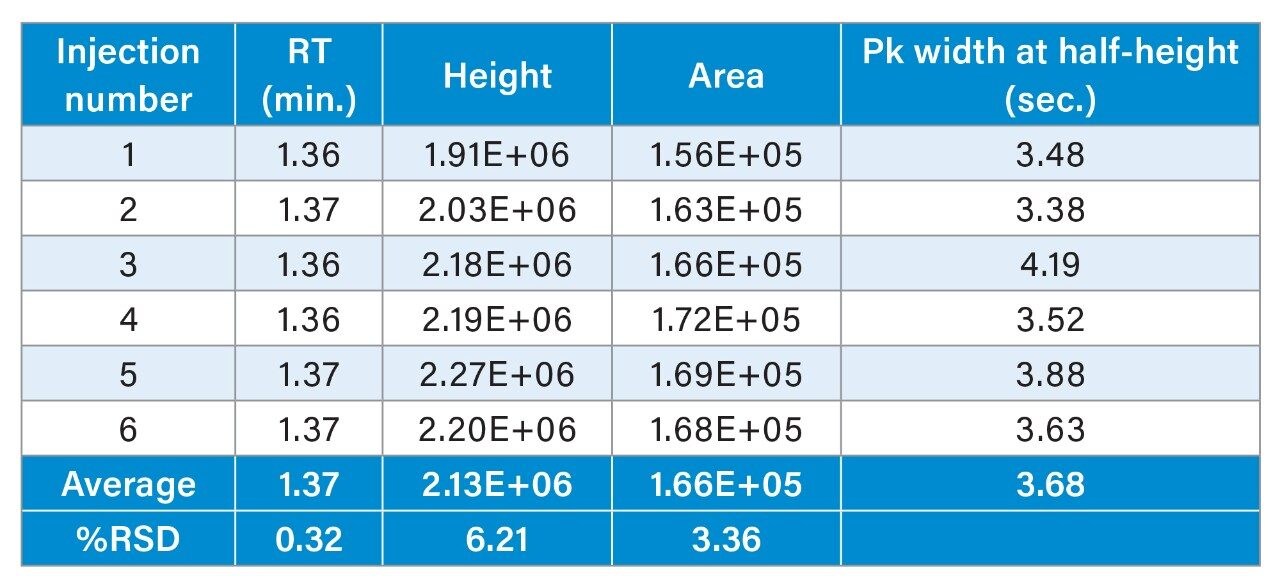
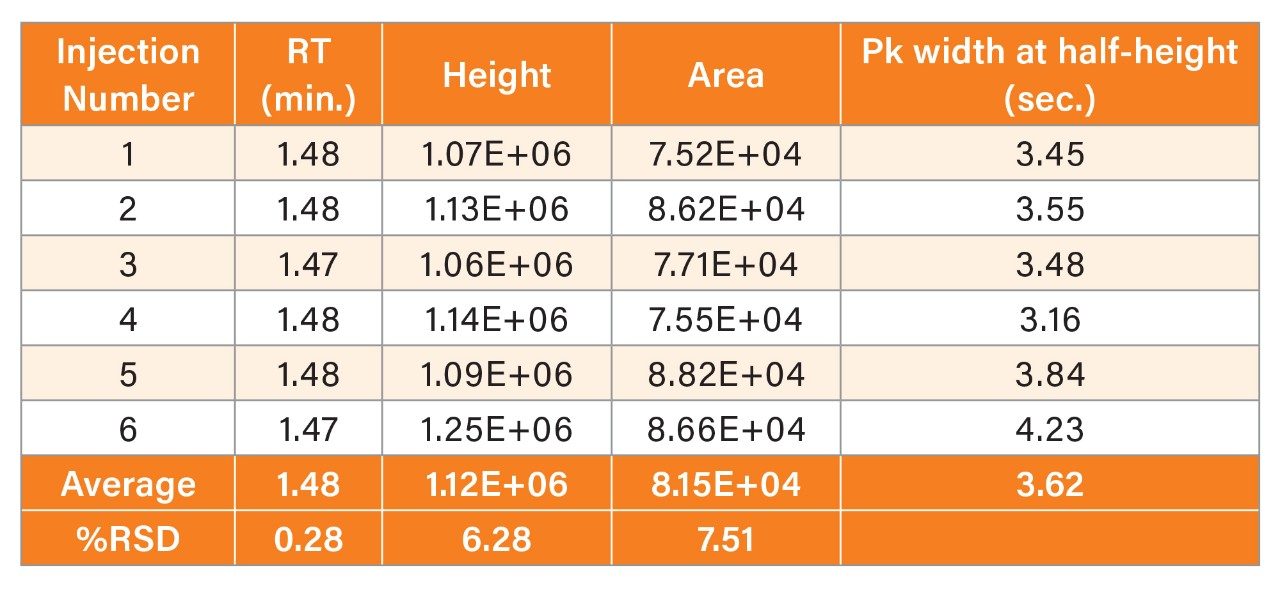
As shown in the chromatograms in Figure 4, methylmalonic acid is retained similarly on both columns and the peak widths are about the same. However, the MaxPeak Premier Column gives superior peak heights and peak areas, with an increase in height of 90% and area by 103% (Tables 1 and 2). This attests to the success of the inert surface in mitigating adsorption of methylmalonic acid on the column hardware. The inert surface mitigates the adsorption of analyte to the hardware, leading to higher recoveries for quantitation.
The MaxPeak Premier Column also demonstrated improved peak area precision. The percent relative standard deviation (RSD) for area was lowered from 7.51% to 3.36%. This is consistent with a reduced impact of adsorption, leading to improved recovery of the analyte and greater confidence in the analytical data.
Small, polar molecules are prevalent compounds not only as substrates in organic syntheses but also as key molecules in metabolic samples in the field of metabolomics. These molecules often contain electron-rich groups such as carboxylates and phosphates and pose a unique challenge in reversed-phase chromatography. The charged-surface hybrid (CSH) Phenyl Hexyl Column, with embedded groups to impart an overall positive charge in acidic mobile phases, successfully retains polar acids such as methylmalonic acid without resorting to other techniques such as HILIC chromatography or the use of ion-pairing and/or derivatization.6
Additionally, MaxPeak Premier Columns were specifically developed to mitigate the adsorption of analytes having electron-rich groups with the metal oxide layer of the column hardware and to minimize the formation of iron adducts. In the analysis of methylmalonic acid, the MaxPeak Premier Column demonstrated a two-fold increase in peak heights and peak areas. For six replicate injections of standard on each column, the MaxPeak Premier Column also showed superior peak area precision, with the relative standard deviation decreased more than two-fold.
As analytical samples and matrices become more complicated, especially in the context of metabolomics and biological fluids analysis, it is challenging to have complete confidence in your data. The inert surface hardware of MaxPeak Premier Columns strongly mitigates interactions between analytes and the column hardware and provides another level of control in the quality and robustness of your chromatographic analysis.
720008748, April 2025