This is an Application Brief and does not contain a detailed Experimental section.
For research use only. Not for use in diagnostic procedures.
Here we describe a clinical research method for the simultaneous analysis of vitamins A and E in serum by single quadrupole mass detector, the ACQUITY QDa. The extraction method is a modification of a previous LC-MS/MS method, using HLB Prime for sample preparation. The combination of a chromatographic method that uses an ACQUITY UPLC HSS PFP Column and an ACQUITY QDa testing under SIR mode provides an accurate and sensitive method for the analysis of the vitamins A and E in serum. The injection cycle is just 5 minutes and the sensitivities could achieve 100 ppb and 1 ppm for vitamins A and E in the serum, respectively.
Historically, the majority of vitamins A and E analysis is performed by HPLC with UV detection. Even when the analysis of vitamin A and E involves LC-MS/MS, the extraction methods used such as liquid-liquid extraction (LLE) suffer from very long extraction times, with high solvent consumption and lengthy analysis time. Previously, we have shown a clinical research method on LC-MS/MS that requires just 100 μl sample volume and provides a shortened analysis time.1 The removal of phospholipid by Oasis HLB PRiME (SPE) is a key factor in this improvement of performance. In this technical brief, we will demonstrate how LC-MS could provide an accurate and sensitive analysis of vitamin A and E from the serum.
Isotopically labelled internal standards were added to the 100 μL serum and then a protein precipitation procedure was performed by the addition of ethanol. After centrifugation, the supernatant was diluted by ethanol/water (5:3) to give a final ethanol/water ratio of 2:3. The resulting solution of 650 μL was applied to Oasis HLB elution plates for sample clean up. The sample was cleaned with 20% ACN (aq) and then eluted in 100% ACN. The eluent was further diluted with water to 200 μL (Figure 1). Taking the advantage of using HLB PRiME, no activation and evaporation step was required. The 20 μL resulting extract was injected to LC-MS for the analysis.
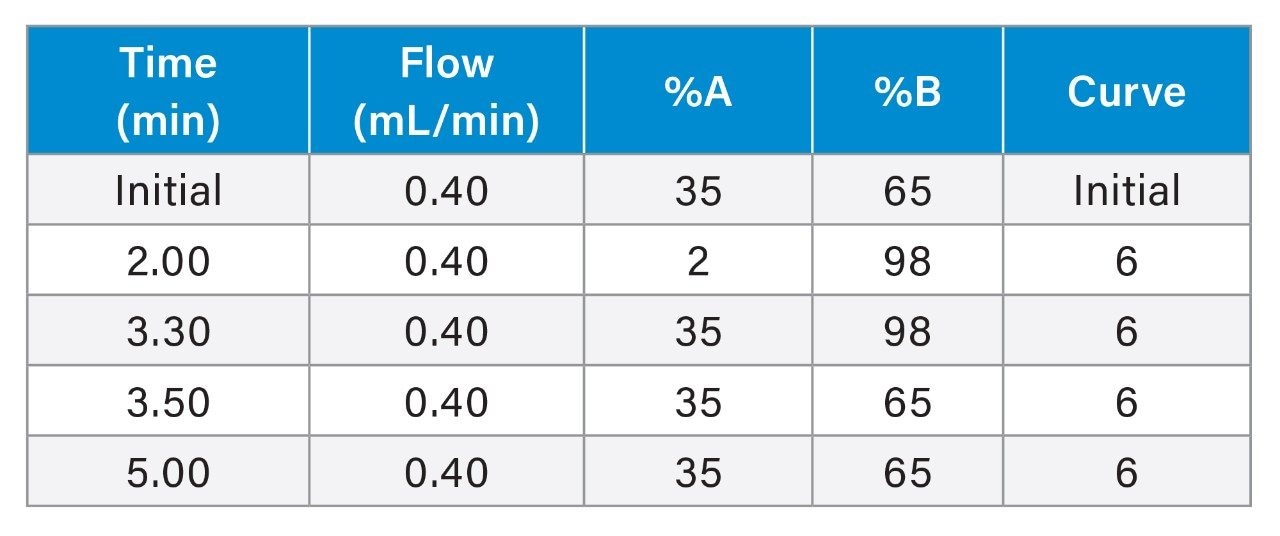
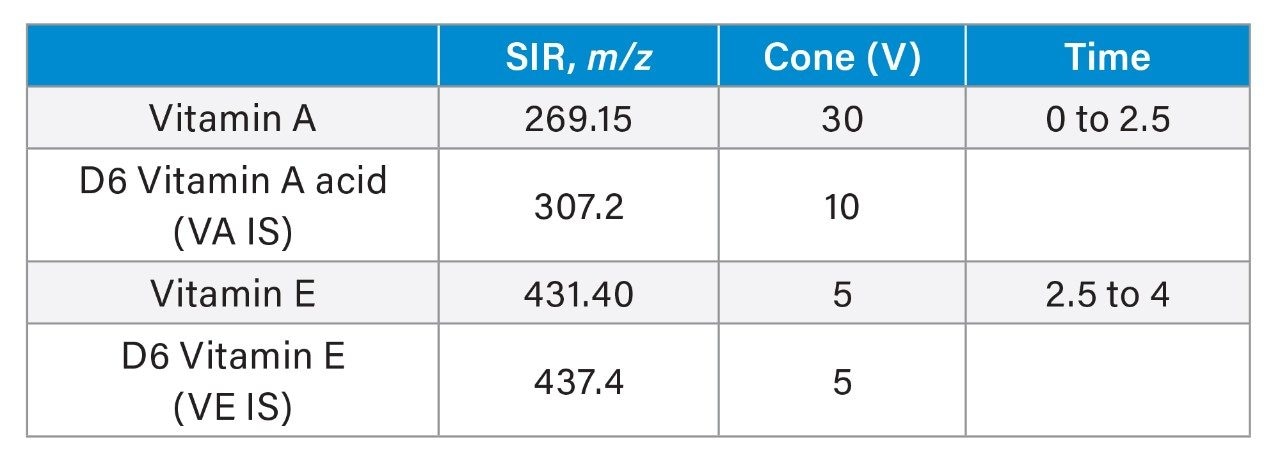
Using an ACQUITY UPLC H-Class PLUS System with an ACQUITY HSS PFP Column (1.8 μm, 2.1 x 50 mm, P/N:186005965), a gradient elution of 65% mobile phase A (MP A) to 100% MP A was run to achieve the separation. Detection was performed by ACQUITY QDa under ESI positive and SIR mode for the corresponding protonated adducts. The injection cycle was 5 minutes.
|
LC system: |
ACQUITY UPLC H-Class Parameter |
|
Vials: |
96-well collection plate containing 1 mL inserts (P/N: 186000855) |
|
Column |
ACQUITY UPLC HSS PFP Column 2.1 x 50 mm, 1.8 µm (P/N: 186005965) |
|
Column temp.: |
40 °C |
|
Sample temp.: |
10 °C |
|
Injection volume: |
20 µL |
|
Flow rate: |
0.40 mL/min |
|
Mobile phase A: |
Water + 2 mM NH4Ac + 0.1% formic acid |
|
Mobile phase B: |
MeOH + 2 mM NH4Ac + 0.1% formic acid |
|
MS system: |
ACQUITY QDa |
|
Ionization mode: |
ESI+ |
|
Acquisition mode |
SIR |
|
Capillary voltage: |
0.8 kV |
|
Cone voltage: |
See channel details |
|
LC-MS software: |
MassLynx v4.2 |
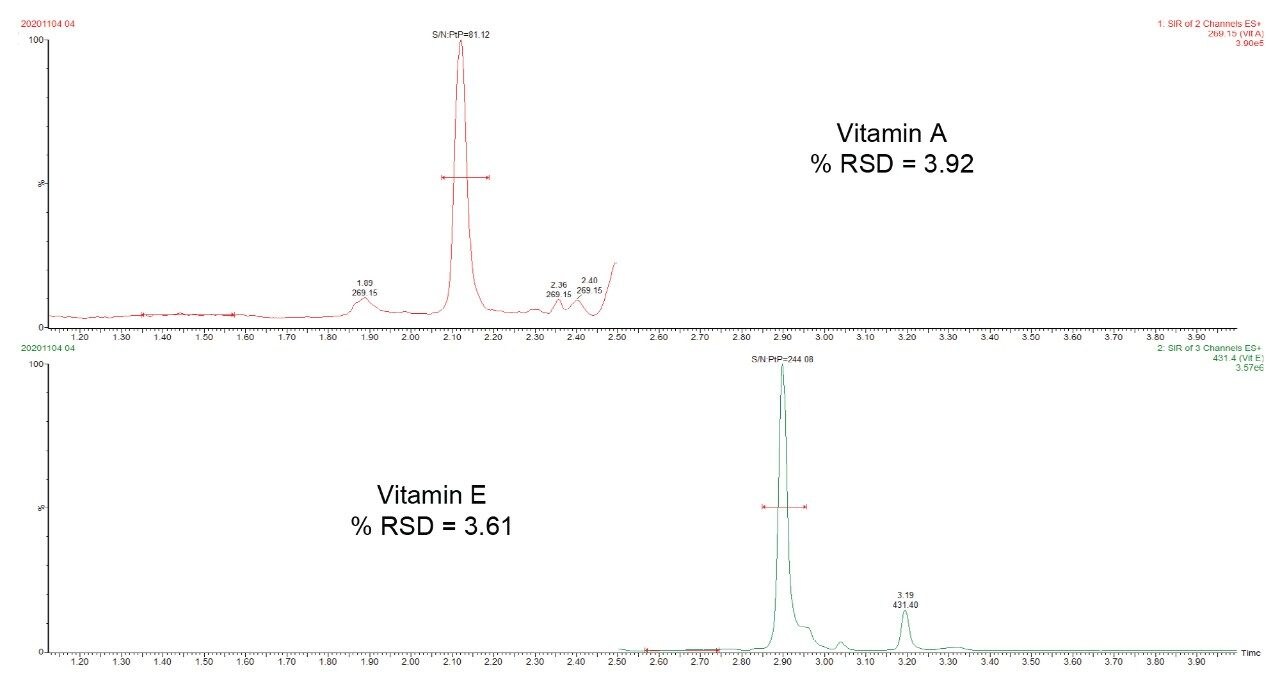
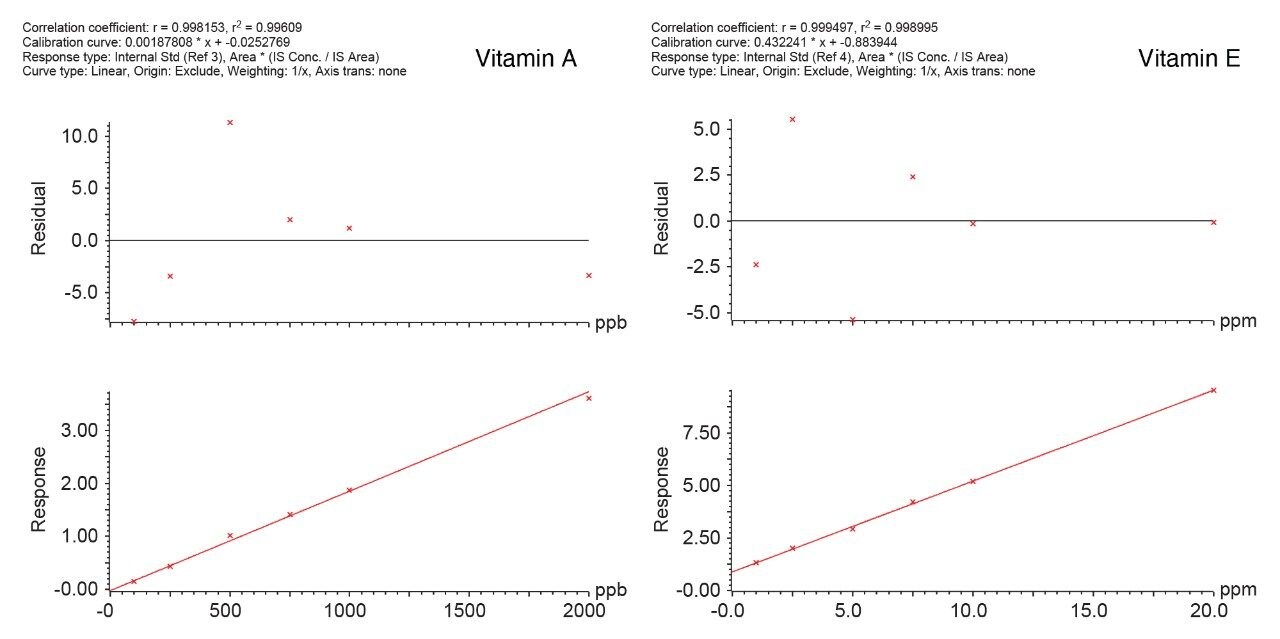
Figure 2 shows the chromatograms of 100 ppb and 1 ppm of vitamin A and E in the stripped serum (MSG2000). The analytical sensitivity investigations demonstrate that quantitation at 100 ppb of vitamin A and 1 ppm of vitamin E are achievable (%RSD <20, Bias <15%, S/N >10). The method was shown to be linear across the range of 100 ppb to 2000 pp for vitamin A and 1 ppm to 20 ppm for vitamin E. Correlation coefficients (r2) >0.993 achieved for both compounds across 10 separate occasions. Example of calibration curves are shown in Figure 2.
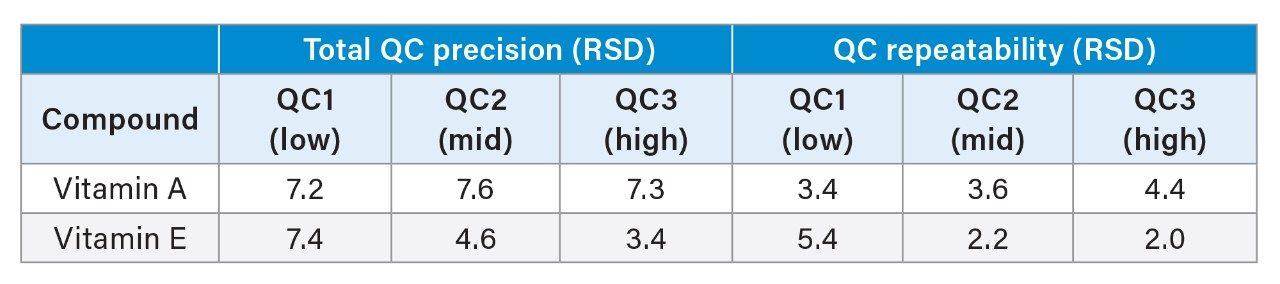
Precision was assessed by extracting and analysing 5 replicates from low to high concentrations in stripped serum over 5 days (n=25). Repeatability and total precision were ≤8% CV at all concentration levels tested for vitamin A and E and they were summarised in Table 1. All calculated % recoveries were within ±15% for typical endogenous interferences tested when comparing test and control samples.
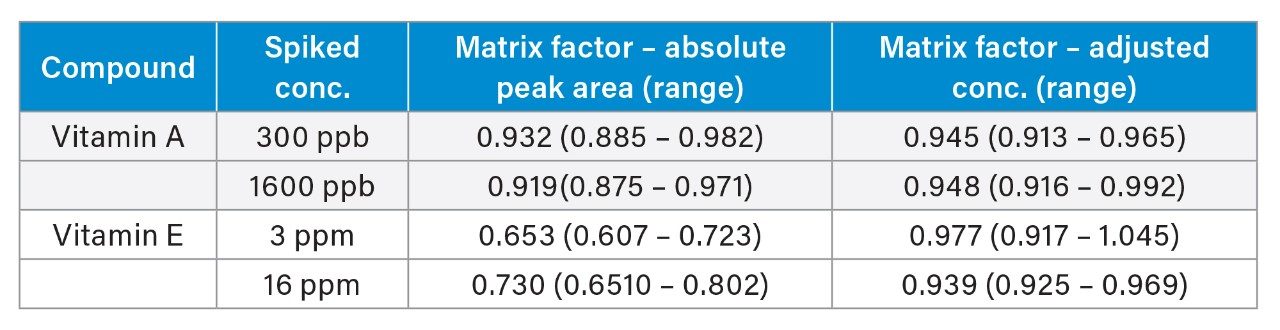
Matrix factor results were within ±15% between test samples from six individuals and control samples compensated for using the internal standard (Table 2). Matrix suppression was observed for vitamin E (mean=0.653 and 0.730), but it was compensated by the internal standard (mean= 0.977 and 0.939).
We explored the application of a UPLC-Single Quadrupole MS System, the ACQUITY UPLC-ACQUITY QDa, on a clinical research analysis of fat-soluble vitamins. The results show that the method is accurate and analytically sensitive even in a complex matrix. The sample preparation is relatively simple compared to LLE and the run time is significant decreased compare to traditional HPLC-UV methods.
720007164, February 2021