This application note describes the analysis of quetiapine using three sample extraction protocols; protein precipitation, Oasis MCX and Oasis PRiME MCX to compare analyte recovery and matrix effects including phospholipid removal.
Bioanalysis plays an essential role in drug discovery and development, of which sample preparation is a critical step in order to achieve reliable results.1 Speed, method robustness and cost are important factors to consider when developing an analytical method for a high-throughput laboratory.
Oasis PRiME MCX, a mixed-mode/strong cation-exchange Solid-Phase Extraction (SPE) product, has been developed to generate cleaner extracts for basic compounds compared to other sample preparation techniques, by the removal of phospholipids. In this application, a fast 3-step protocol, that eliminates the conditioning and equilibration steps compared to conventional Solid Phase Extraction (SPE), was used to extract quetiapine from human plasma in a 96-well plate µElution format.
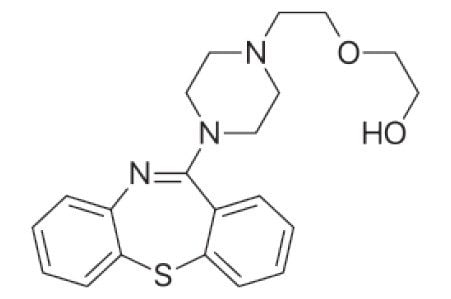
The structure of quetiapine is shown in Figure 1. Quetiapine is a dibenzothiazepine derivative and antipsychotic agent used for the treatment of a wide variety of psychotic disorders. This basic drug (pKa 7.06 of the amine) is ideal for sample extraction using a cation exchange sorbent.
This application note describes the analysis of quetiapine using three sample extraction protocols; protein precipitation, Oasis MCX and Oasis PRiME MCX to compare analyte recovery and matrix effects including phospholipid removal.
In-house calibrators were prepared by spiking quetiapine (Sigma Aldrich, Dorset, England) into pooled human plasma (Sera Laboratories, West Sussex, United Kingdom) over the concentration range 2.5–100 ng/mL. QC samples were also prepared in-house at 7.5, 30, and 75 ng/mL.
100 µL of plasma calibrator/QC was transferred to a graduated microfuge tube. The proteins were precipitated by the addition of 300 µL of methanol. Samples were capped and vortexed for 30 seconds prior to centrifugation for 5 minutes at 13000 rpm.
100 µL of plasma calibrator/QC was transferred to a graduated microfuge tube. To disrupt protein binding and protonate the analyte to enable ion exchange interactions, 100 µL of 4% phosphoric acid was added to each sample. The samples were capped and vortexed for 30 seconds prior to centrifugation for 5 minutes at 13000 rpm. The supernatant was transferred to a Oasis MCX 96-well µElution Plate that had been conditioned with methanol and equilibrated with water. The plate was washed with 2% formic acid (aqueous) followed by 100% methanol. Samples were eluted with 2 x 25 µL of 5% ammonium hydroxide in methanol followed by 50 µL of water. The plate was sealed and lightly vortexed for 30 seconds before analysis.
100 µL of plasma calibrator/QC was transferred to a graduated microfuge tube. To disrupt protein binding and protonate the analyte to enable ion exchange interactions, 100 µL of 200 mM ammonium formate with 4% phosphoric acid was added to each sample. The samples were capped and vortexed for 30 seconds prior to centrifugation for 5 minutes at 13000 rpm. The supernatant was transferred to a Oasis PRiME MCX 96-well µElution plate and washed with 100% methanol. Samples were eluted with 2 x 25 µL of 5% ammonium hydroxide in methanol followed by 50 µL of water. The plate was sealed and lightly vortexed for 30 seconds before analysis.
The Solid-Phase extraction procedures are summarized in Figure 2.

|
LC system: |
ACQUITY UPLC I-Class System (FTN) |
|
Column: |
ACQUITY UPLC CSH C18 1.7 μm, 2.1 x 100 mm (p/n 186005297) |
|
Mobile phase A: |
Water with 0.1% formic acid |
|
Mobile phase B: |
Methanol |
|
Wash solvent: |
80% methanol(aq) and 0.1% formic acid |
|
Purge solvent: |
20% methanol(aq) and 0.1% formic acid |
|
Seal wash: |
20% methanol(aq) |
|
Column temp.: |
45 °C |
|
Sample temp.: |
10 °C |
|
Injection vol.: |
5 μL |
|
Flow rate: |
0.4 mL/min |
|
Gradient: |
See table 1 |
|
Run time: |
4.3 minutes (approximately 5.0 minutes injection to injection) |

|
System: |
Xevo TQD |
|
Resolution: |
MS1 and MS2 (0.75 FWHM) |
|
Acquisition mode: |
Multiple Reaction Monitoring (MRM) (see table 2 for details) |
|
Polarity: |
ESI positive |
|
Capillary voltage: |
0.5 kV |
|
Cone voltage: |
See table 2 |
|
Source temp.: |
150 °C |
|
Desolvation temp.: |
600 °C |
|
Dwell time: |
0.05 seconds |
|
Inter-scan delay: |
0.02 seconds |
|
Inter-channel delay: |
0.01 seconds |

MassLynx v4.1 with TargetLynx Application Manager
The extraction recovery for protein precipitation, Oasis MCX, and Oasis PRiME MCX sample extraction were determined by the analysis of three samples across the concentration range in replicates of 6. The extraction recovery was calculated using the following equation:
%Recovery = (Area A / Area B) x 100
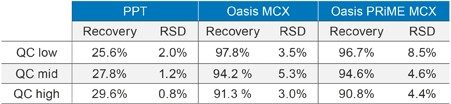
Where A is the peak area of an extracted sample and B is the peak area of an extracted matrix sample in which quetiapine was added post-extraction. Excellent extraction recovery and precision was observed across the concentration range for quetiapine using Oasis MCX (mean 94.5% recovery) and Oasis PRiME MCX (mean 94.0% recovery), however, very poor recovery (mean 27.7% recovery) was observed for the protein precipitation method (Table 3).
The matrix effects for protein precipitation, Oasis MCX, and Oasis PRiME MCX sample extraction were determined by the analysis of three samples across the concentration range in replicates of 6. The matrix effects were calculated using the following equation:
Matrix effects = ((Peak area in the presence of matrix / Peak area in teh absence of matrix)-1) x 100
The peak area in the presence of matrix refers to an extracted matrix sample in which quetiapine was added post-extraction. The peak area in the absence of matrix refers to a solvent solution of quetiapine.
There was significant matrix effects observed when using protein precipitation; the mean matrix effect was -58.4% (range -56.4% to -59.6%) indicating severe ion suppression. Using mixed-mode/strong cation-eXchange Solid-Phase extraction the matrix effects observed were negligible, Oasis MCX mean matrix effect was -2.7% (range 0 to -4.3%) and Oasis PRiME MCX mean matrix effect was -0.1% (range -1.9% to 1.2%).
To assess phospholipid removal a parent ion scan experiment was conducted monitoring parent ions of m/z 184 in plasma sample extracts using the three extraction protocols. The phospholipid peak areas were normalized to the protein precipitation extract, which contained the highest levels. Figure 3 shows the comparative phospholipid levels in plasma sample extracts from protein precipitation, Oasis MCX and the 3-step Oasis PRiME MCX Protocol demonstrating phospholipid removal of up to 98% for the 3-step Oasis PRiME MCX Protocol when compared to protein precipitation.

Oasis PRiME MCX and the 3-step protocol removes 98% of phospholipids compared to protein precipitation, whilst, providing reproducible results and cleaner extracts with negligible matrix effects.
Even though protein precipitation is an inexpensive form of sample preparation, the matrix effects indicate that inefficiencies of the sample clean-up which may lead to poor robustness of the bioanalytical method.
The µElution format and 3-step protocol enables a faster workflow, through the use of a simplified protocol and direct injection of the plasma extract without the need for evaporation or reconstitution. This SPE format and extraction protocol can be easily automated using a liquid handling robot to improve laboratory workflow, eliminate transcription errors and allow for sample tracking capabilities.
720006191, January 2018