
The purpose of this work is to demonstrate a direct injection method for the determination of acrylamide and haloacetic acids in drinking water that exceeds the requirements for the new EU Drinking Water Directive 2020. The method performance study was completed on an ACQUITY UPLC I-Class PLUS with a Xevo TQ-S micro using a reversed-phase LC method. Method performance was assessed using 3 spike levels (2, 4, and 30 µg/L) for 9 haloacetic acids and acylamide (0.02, 0.04, and 0.08 µg/L) with 22 replicates at each level in mineral water preserved with ammonium chloride additive. All average recoveries were between 97 to 102% with RSDs all less than 8% for analytes at each spike level. This was achieved using a short LC-MS/MS run time of under 8 minutes and a 10 µL injection. Repeatability was tested with 270 injections of a 2 µg/L haloacetic acids and 0.1 µg/L acrylamide mineral water calibration standard with no user intervention. Results showed RSDs less than 10% for peak area response and RSDs less than 1% for retention time for all analytes tested. From the method performance study, limits of quantification (LOQ) for individual haloacetic acids was 0.5 µg/L and 0.02 µg/L for acrylamide based on the lowest calibration standard. These LOQs exceed the requirements of the new EU Drinking Water Directive of 60 µg/L for the sum of MCAA, DCAA, TCAA, MBAA, and DBAA and 0.1 µg/L for acrylamide.
The increased focus in the haloacetic acids is derived from their risk as potential human carcinogens1 and are formed during the water disinfection process in the presence of organic material. Acrylamide is a recognized human carcinogen2 and can be present in drinking water through contamination from grouting in the water network (it is present in grouting agents) and in flocculants for the clarification of potable water.
A fast, efficient method for the determination of haloacetic acids and acrylamide contaminants in drinking water is important as we recognize and manage risks associated with chemical contaminant in drinking water supplies. The World Health Organization (WHO) has updated guidelines in 20173 and the EU are to set new standards in chemical contamination in an updated EU Drinking Water Directive.4 The new Directive includes MCAA, DCAA, TCAA, MBAA, and DBAA (with a parametric value of 60 µg/L) plus identifies that continued monitoring of acrylamide is still required (with a parametric value of 0.1 µg/L). The US EPA includes 5 haloacetic acids (HAA MCL = 60 µg/L) and acrylamide (zero tolerance) in their National Primary Drinking Water Regulations.5
Common methods for the analysis of haloacetic acids require either sample preparation or derivatization with determination by GC-ECD, GC-MS(/MS) or by direct injection onto an Ion Chromatography-MS(/MS) System.6, 7, 8 We have developed a direct injection method with low injection volume utilizing Reversed Phase Liquid Chromatography coupled to a Tandem Quadrupole Mass Spectrometer that includes both haloacetic acids and acrylamide. This approach reduces the need for either sample preparation and/or derivatization and decreases risk of potential analyte losses associated with each. The method also eliminates the use of HILIC with the associated long column re-equilibration times and removes the need for the addition of ion chromatography separation systems.
Several 1 liter samples of tap water from a soft water (Wilmslow, UK) and hard water (York, UK) area were collected and transported to the applications laboratory (Wilmslow, UK). Several 1 liter samples of a generic mineral water were purchased in the UK for use as matrix blanks.
Once the drinking water samples were received in the laboratory. They were split into aliquots of 50 mL with 5 mg of ammonium chloride added as a preservative.1,7 The samples were then stored at 4 °C in a fridge until analysis.
Just prior to analysis, 50 µL of formic acid was added to the 50 mL sample. An aliquot of the resulting mix was then transferred to a 2 mL LC vial where isotopically labelled internal standard was then added (acrylamide d3) for the determination of acrylamide. The haloacetic acids were calibrated externally and acrylamide-d3 was used as an internal standard for the acrylamide only.
After the method development phase, a method performance study was conducted which consisted of 2 batches containing 33 spiked mineral water samples: 11 spikes at a designated low, mid and high concentration (2, 4, and 30 µg/L) for the haloacetic acids and (0.02, 0.04, and 0.08 µg/L) for acrylamide. A matrix effect assessment was conducted in the first batch by running calibration standards in both LC-MS grade water and mineral water.
|
LC system: |
ACQUITY UPLC I-Class PLUS |
|
Vials: |
TruView LCMS Certified Clear Glass 12 x 32 mm, Screw Neck Vial (p/n: 186005666CV) |
|
Column(s): |
ACQUITY UPLC HSS C18 SB 1.8 µm, 2.1 x 100 mm (p/n: 186004119) |
|
Column temp.: |
30 °C |
|
Sample temp.: |
10 °C |
|
Injection volume: |
10 µL |
|
Flow rate: |
0.4 mL/min |
|
Mobile phase A: |
Water with 0.05% acetic acid (LC-MS grade) |
|
Mobile phase B: |
Methanol with 0.05% acetic acid (LC-MS grade) |
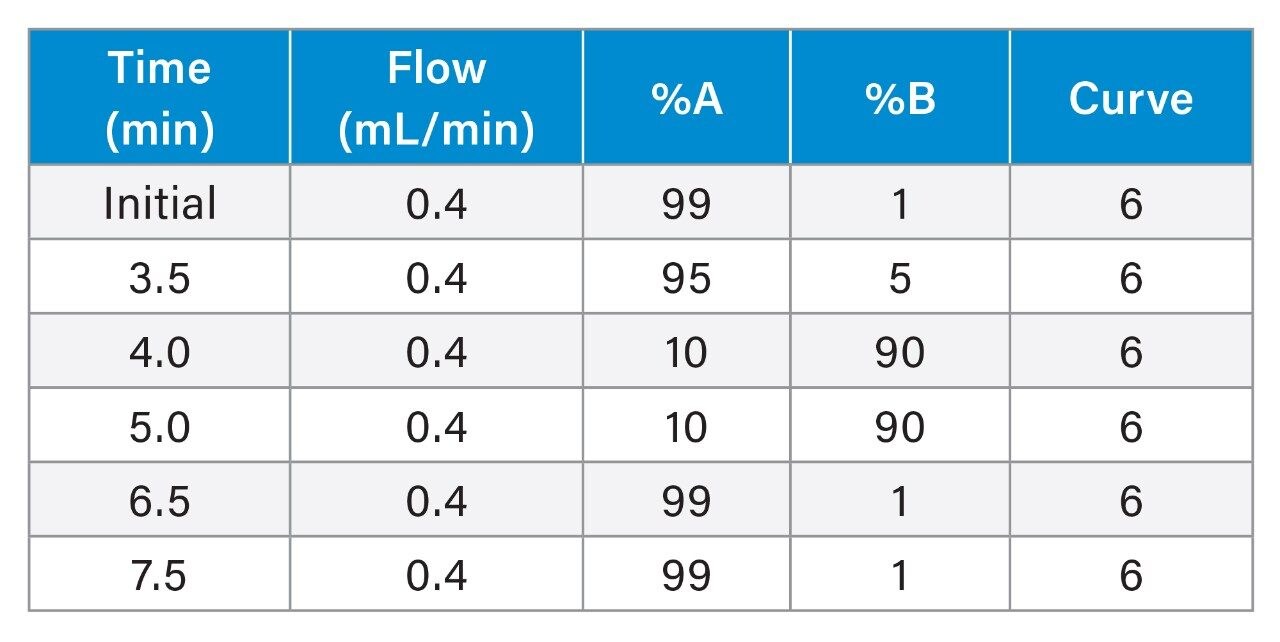
|
MS System: |
Xevo TQ-S micro |
|
Ionization mode: |
ESI+ for acrylamide, ESI- for HAAs |
|
Acquisition range: |
MRM |
|
Capillary voltage: |
0.5 kV for both polarities |
|
Cone gas flow: |
50 L/hr |
|
Desolvation temp.: |
600 °C |
|
Desolvation gas flow: |
1000 L/hr |
|
Source temp.: |
150 °C |
|
Informatics: |
MassLynx v4.2 |
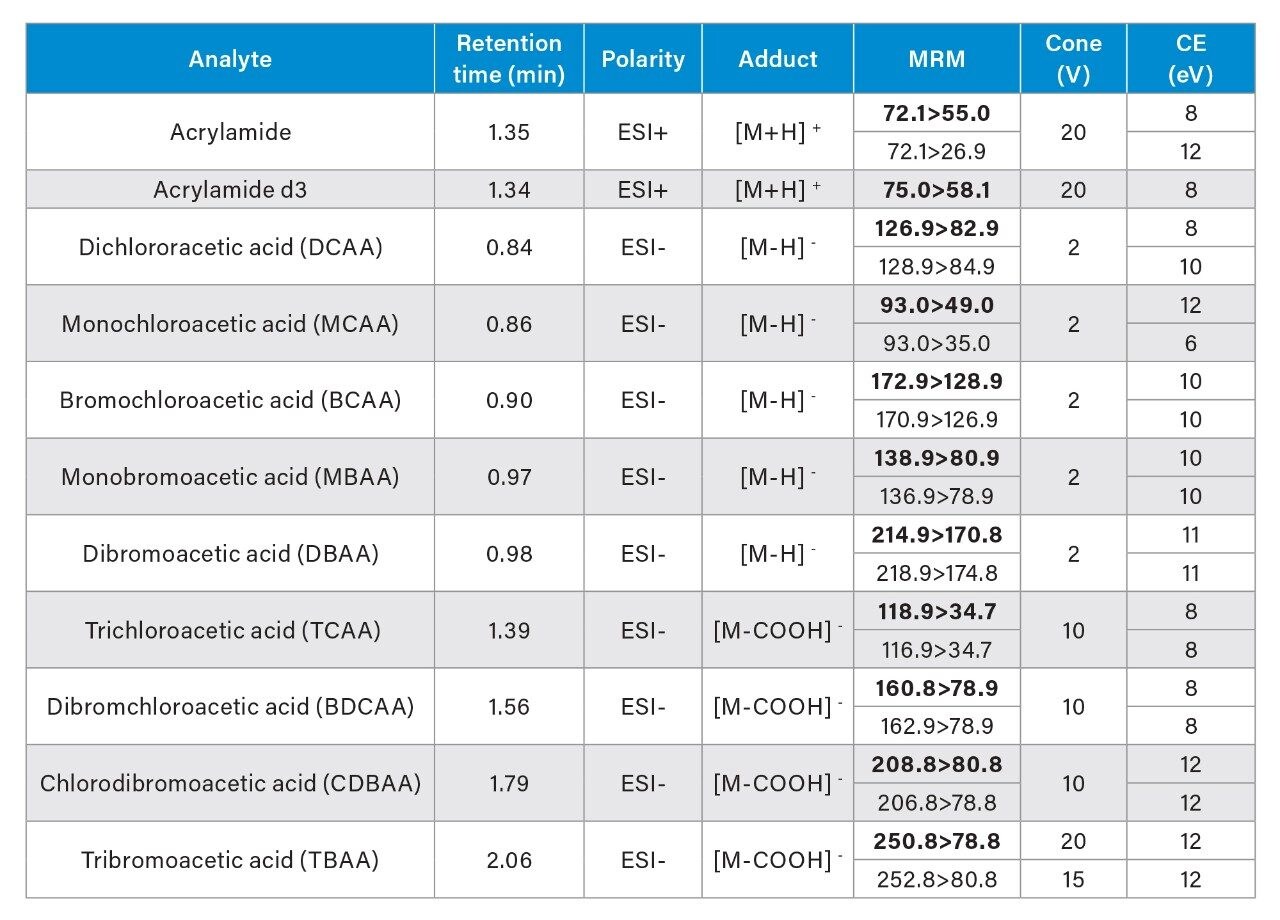
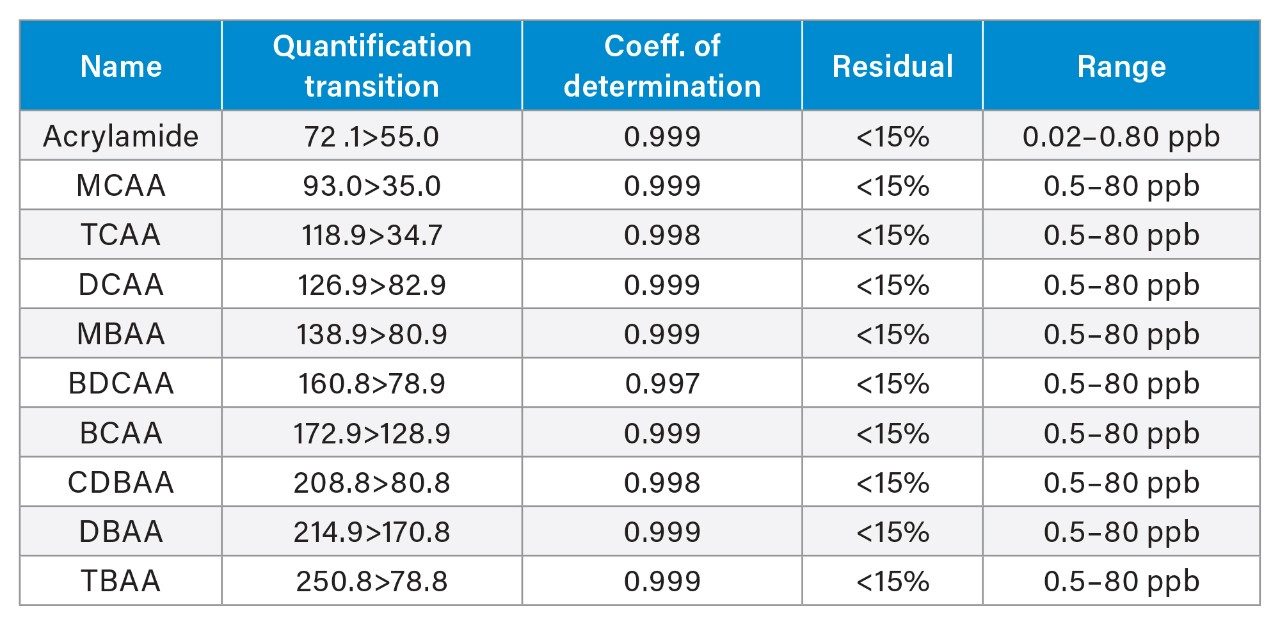
The MRMs listed in Table 1 highlight the optimized transitions used for quantification and confirmation of the haloacetic acids and acrylamide in this application. The protonated parent ion was identified for acrylamide and deprotonated parent ions were identified for MCAA, DCAA, MBAA, BCAA, and DBAA. For TCAA, BDCAA, CDBAA, TBAA (M-COOH)- was identified as a suitable parent ion fragment for use. From the mass spectra produced from infusion experiments, stable in-source fragments were identified and taken forward to generate fragment ions. The results presented in this application note highlights that this is a valid approach for these haloacetic acids based on the results presented and is a common approach reported in literature for the haloacetic acids.1,6
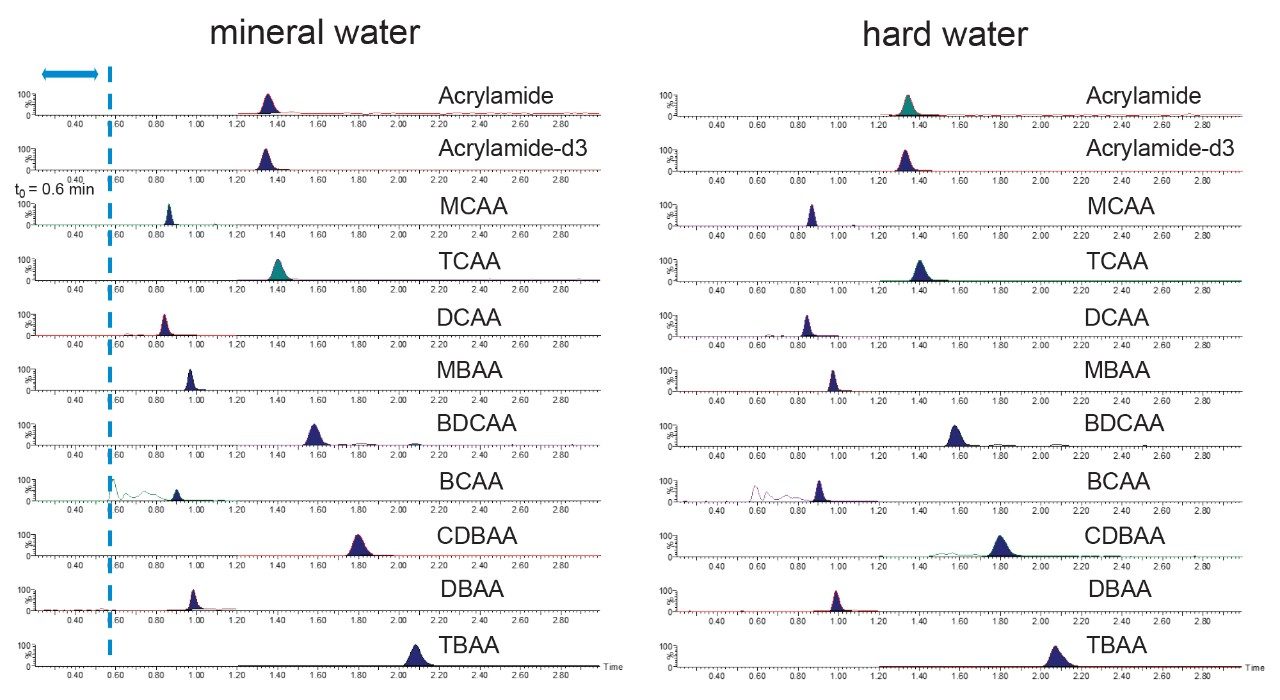
The drinking and mineral water samples collected were screened for the presence of haloacetic acids and acrylamide before the method performance study was started. Acrylamide was not detected in any of the water samples. No haloacetic acids were detected in the mineral water. For the soft water only TBAA was not detected, with the remaining 8 haloacetic acids quantified by standard addition found to be present with concentrations ranging from 0.33 to 11.1 µg/L. Hard water only tested positive for DBAA at 0.44 µg/L with quantification by standard addition. As the levels detected in both the soft and hard drinking water samples would affect the method performance study, the mineral water was selected as a representative sample type. To simulate sample collection by water companies, ammonium chloride (preservative) was added to all samples (and calibration standards) with formic acid being added just before analysis to reflect current practice.
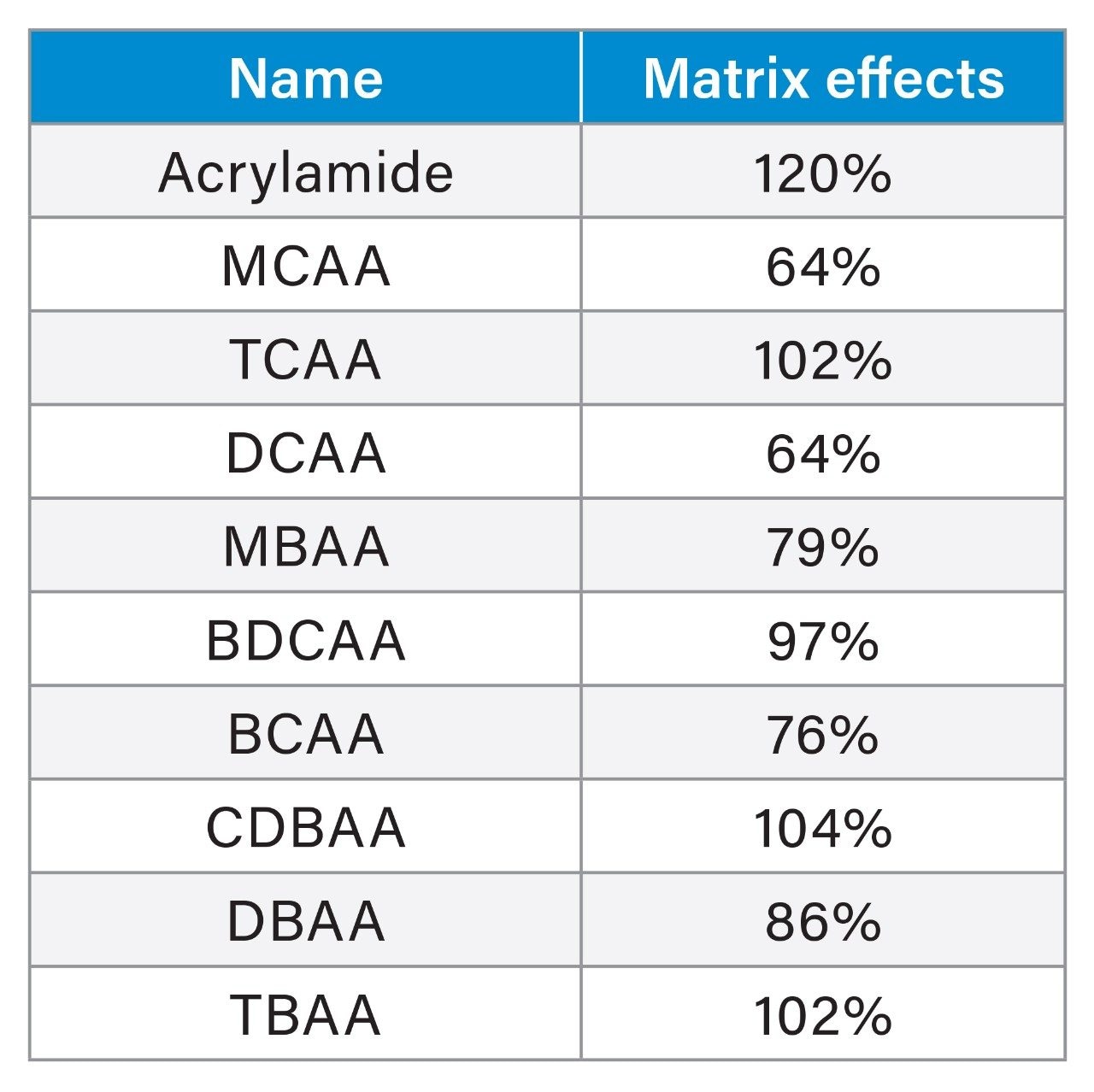
The effect of matrix was investigated on the response of the analytes. Table 2 highlights the difference in results obtained when quantifying against “solvent” standards (in this case LC-MS grade water) and matrix standards (mineral water). Therefore, matrix matching the calibration standards to the type of water is an important operational aspect to implement with this approach.
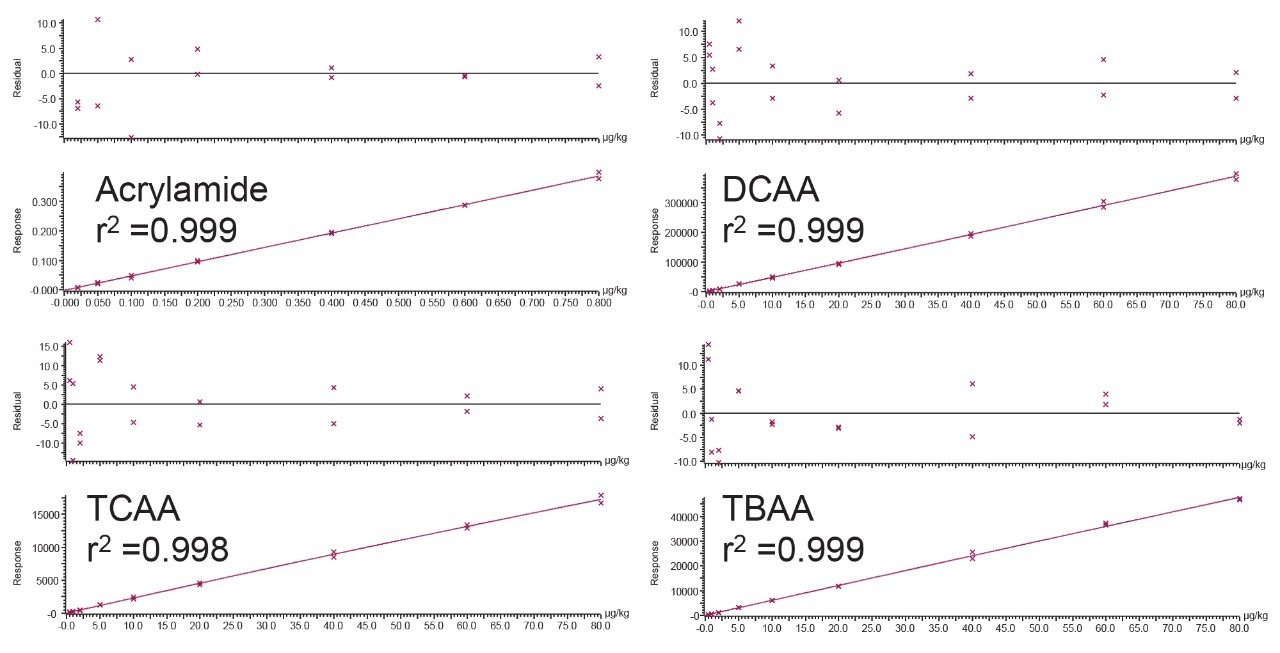
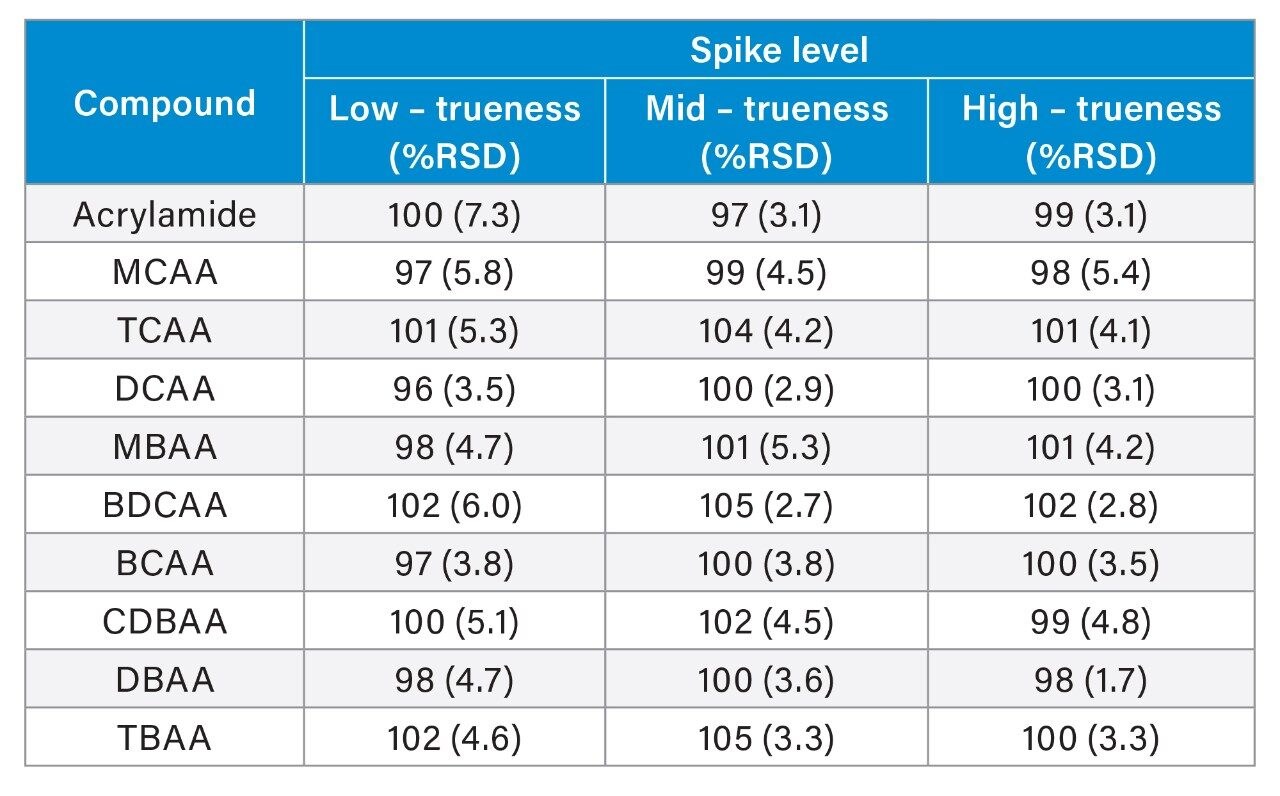
Method performance was assessed over 2 analytical batches, where each batch was comprised of 11 spikes at designated low, mid, and high levels for each of the haloacetic acids (2, 4, and 30 µg/L respectively) and acrylamide (0.02, 0.04, and 0.08 µg/L respectively). Mineral water, treated as described in the experimental section, was used throughout the method performance study. Matrix matched calibration standards in mineral water were prepared over the range of 0.5–80 µg/L for the haloacetic acids and 0.02–0.8 µg/L for acrylamide. Figure 1 demonstrates expected calibration graphs from this approach. All coefficients of determination were greater than 0.996 and residuals from the calibration graphs were all lower than 15%, Table 3 shows calibration performance observed. Results from the method performance batches are presented in Table 4 which highlights the method performance as suitable for the quantification of the haloacetic acids and acrylamide in drinking water. An example of the chromatography achieved at low spike level for all compounds in the mineral water spikes (verses the standard addition in hard water) is displayed in Figure 2. From the method performance study, limits of quantification (LOQ) for individual haloacetic acids were 0.5 µg/L and 0.02 µg/L for acrylamide based on the lowest calibration level injected. A sensitivity study conducted before the method performance study highlighted detection of several HAAs at concentrations of 0.05 µg/L. These LOQs exceed the requirements of the new EU Drinking Water Directive (5) of 60 µg/L for the sum of MCAA, DCAA, TCAA, MBAA, and DBAA and 0.1 µg/L for acrylamide.
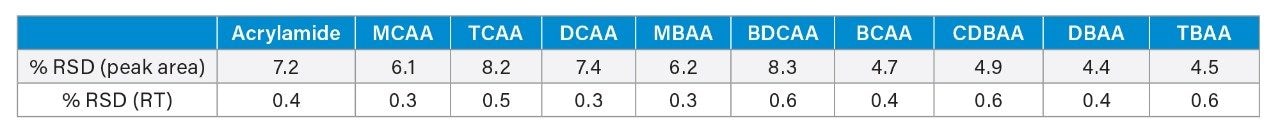
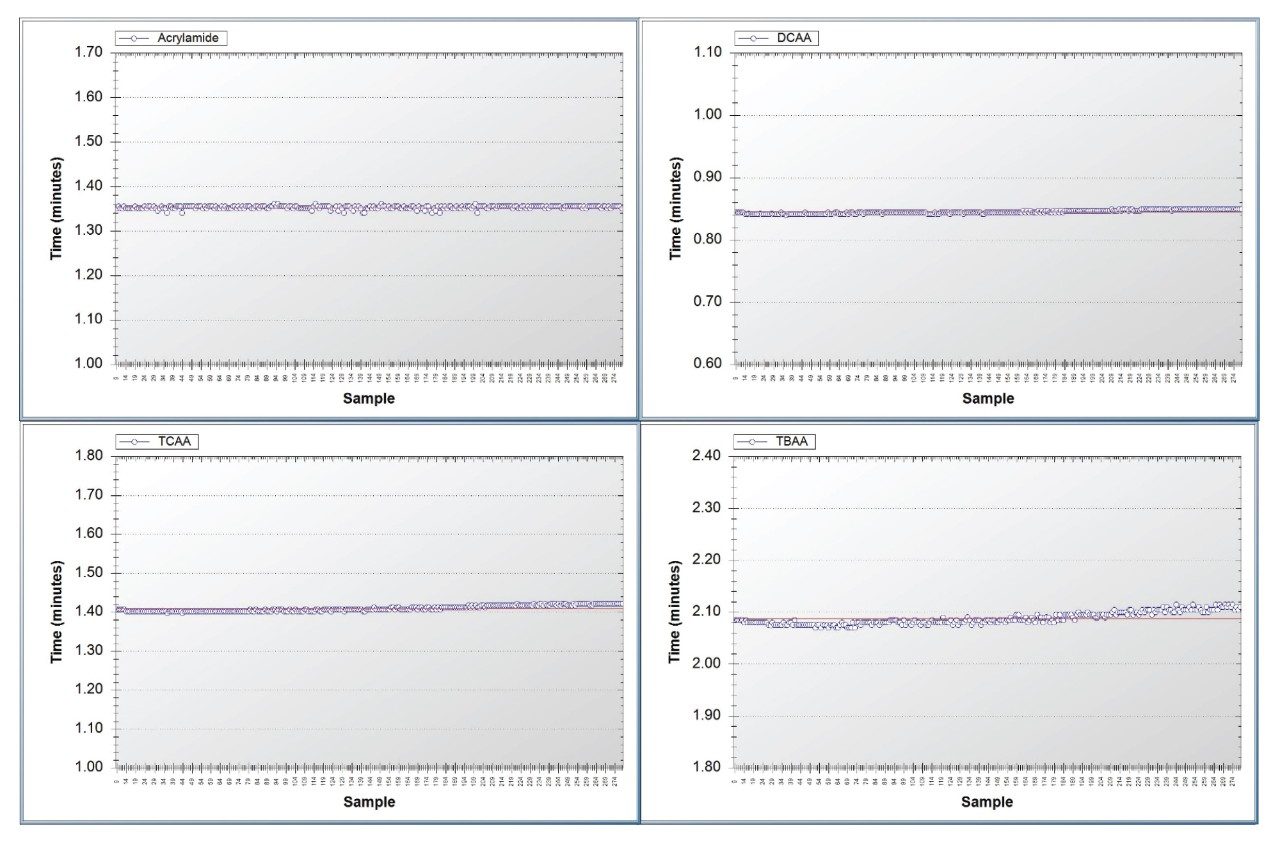
Response and retention time stability are important factors to investigate during a method verification process. To this end a repeatability study of 270 injections of a mineral water matrix standard at 2 µg/L haloacetic acids and 0.1 µg/L acrylamide were run continuously on the method presented. Peak area response variation across all analytes was below 10% RSD, with retention time stability less than 0.7% RSD for all analytes. These results are detailed in Table 5 with the retention time plot for selected analytes presented in Figure 3.
The method performance study data demonstrates that the method for the determination of haloacetic acids and acrylamide by direct injection of water samples onto an ACQUITY UPLC I-Class PLUS coupled to a Xevo TQ-S micro exceeds new requirements in the EU Drinking Water Directive coming into effect in late 2020.4 Limits of quantification for all 9 haloacetic acids was 0.5 µg/L where the directive states the legal limit is 60 µg/L for the sum of MCAA, DCAA, TCAA, MBAA, and DBAA and 0.1 µg/L for acrylamide. The combination of acrylamide into the method increases the scope of the method and improves laboratory efficiency. By using a reversed phase LC approach, potential problems when using HILIC separations have been removed allowing a direct injection approach to be taken. In combination of using a standard UPLC-MS/MS system, this analysis removes the need for specialist LC or IC equipment and method robustness over 270 injections and demonstrates the ability of the method to continually meet required reporting levels over the course of at least 2 days without user intervention. Scientists must validate the method in their own laboratories and demonstrate that the performance is fit for purpose and meets the needs of the relevant analytical control assurance system.
720007071, November 2020