
In this study, sample preparation, cleanup, and analysis protocols were developed for tandem LC-MS determination of a wide variety of veterinary drug residues in seafood tissue samples. This cleanup protocol was effective for removal of both fats and phospholipids. Two types of tissue samples, shrimp (prawn) and salmon, were chosen to demonstrate the suitability of the methodology. Samples were treated with an acidified acetonitrile/water solvent to precipitate proteins and to extract the veterinary drugs of interest. Then, a simple cleanup was performed using a novel SPE device, the Oasis PRiME HLB Cartridge. Representative compounds were chosen from major classes of veterinary drugs including tetracyclines, fluoroquinolones, sulfonamides, macrolides, beta-lactams, NSAIDS, steroids and beta-andrenergics. These compounds were spiked into the seafood samples prior to extraction and cleanup.
In order to insure public health and safety, reliable analytical methods are necessary to determine veterinary drug residue levels in edible tissue samples such as fish and shellfish. The compounds of interest range from highly polar water-soluble compounds to very non-polar fat-soluble compounds. In order to maximize throughput and minimize costs it is desirable to determine the widest possible range of veterinary drug residues in tissue samples with a single analytical method. Seafood and meat tissue for human consumption typically contains up to 20% fat and up to 3% phospholipid.
The major constituents of a typical meat sample are water (up to 70%), protein (15–25%), fat (5–25%) and phospholipid (1–3%). During the sample pre-treatment, the protein is removed from the extract by precipitation and centrifugation. However, significant amounts of fat and phospholipid are co-extracted along with the target veterinary drugs. The presence of these co-extracted substances can lead to interference in the LC-MS analysis, contamination of the analytical column and other components of the UPLC System, and contamination of the mass spectrometer itself. Fats have traditionally been removed from tissue extracts using cumbersome hexane defatting steps or by the use of reversed-phase sorbents such as C18-silica. Although these techniques may be effective for fat removal, neither of these procedures removes phospholipids. In this study, sample preparation, cleanup, and analysis protocols were developed for tandem LC-MS determination of a wide variety of veterinary drug residues in seafood tissue samples. This cleanup protocol was effective for removal of both fats and phospholipids. Two types of tissue samples, shrimp (prawn) and salmon, were chosen to demonstrate the suitability of the methodology. Samples were treated with an acidified acetonitrile/water solvent to precipitate proteins and to extract the veterinary drugs of interest. Then, a simple cleanup was performed using a novel SPE device, the Oasis PRiME HLB Cartridge. Representative compounds were chosen from major classes of veterinary drugs including tetracyclines, fluoroquinolones, sulfonamides, macrolides, beta-lactams, NSAIDS, steroids and beta-andrenergics. These compounds were spiked into the seafood samples prior to extraction and cleanup.
|
LC system: |
ACQUITY UPLC I-Class |
|
Column: |
ACQUITY UPLC CSH C18, 1.7 μm, 100 mm x 2.1 mm ID |
|
Mobile phase A: |
0.1% formic in water |
|
Mobile phase B: |
0.1% formic acid in acetonitrile |
|
Injection vol.: |
5 μL |
|
Injection mode: |
partial loop injection |
|
Column temp.: |
30 °C |
|
Weak needle wash: |
10:90 acetonitrile: water (600 μL) |
|
Strong needle wash: |
50:30:40 water: acetonitrile:IPA (200 μL) |
|
Seal wash: |
10:90 acetonitrile: water |
|
Time (min) |
Flow (mL/min) |
%A |
%B |
Curve |
|---|---|---|---|---|
|
Initial |
0.4 |
85 |
15 |
Initial |
|
2.5 |
0.4 |
60 |
40 |
6 |
|
3.9 |
0.4 |
5 |
95 |
6 |
|
4.9 |
0.4 |
5 |
95 |
6 |
|
5 |
0.4 |
85 |
15 |
6 |
|
7 |
0.4 |
85 |
15 |
6 |
|
Mass spectrometer: |
Xevo TQ-S |
|
Positive ion electrospray (negative ion for chloramphenicol) |
|
|
Source temp.: |
150 °C |
|
Desolvation temp.: |
500 °C |
|
Desolvation gas flow: |
1000 L/Hr |
|
Cone gas flow: |
30 L/Hr |
|
Collision gas flow: |
0.15 mL/Min |
|
Data management: |
MassLynx. v4.1 |
Table 1 summarizes the MRM transitions and instrument parameters used for this study. Also presented in Table 1 are typical matrix matched calibration data for each compound (calculated using the primary transition in shrimp matrix; salmon data were similar) and retention times (RT).
Place a 2.5 g sample of homogenized tissue into a 50 mL centrifuge tube. For standards or QC samples spike with appropriate amounts of desired analytes. Add 10 mL 0.2% formic acid in 80:20 acetonitrile/water. Vortex for 30 seconds and place on mechanical shaker for 30 minutes. Centrifuge at 12000 rpm for 5 minutes.
Note: The extraction/precipitation step gives good recovery of most compounds of interest but also extracts significant amounts of fat and phospholipid.
An Oasis PRiME HLB Cartridge (3cc, 60mg) was mounted on a pre-cleaned vacuum manifold. Cartridge conditioning is NOT required, and was NOT performed. The vacuum was set to 1–2 psi. Approximately 0.5 mL of the supernatant was passed-through the Oasis PRiME Cartridge and collected. A 0.3 mL aliquot of the pass-thru cleanup sample was taken and diluted three-fold with aqueous 10 mM ammonium formate buffer (pH 4.5) prior to UPLC-MS/MS analysis.
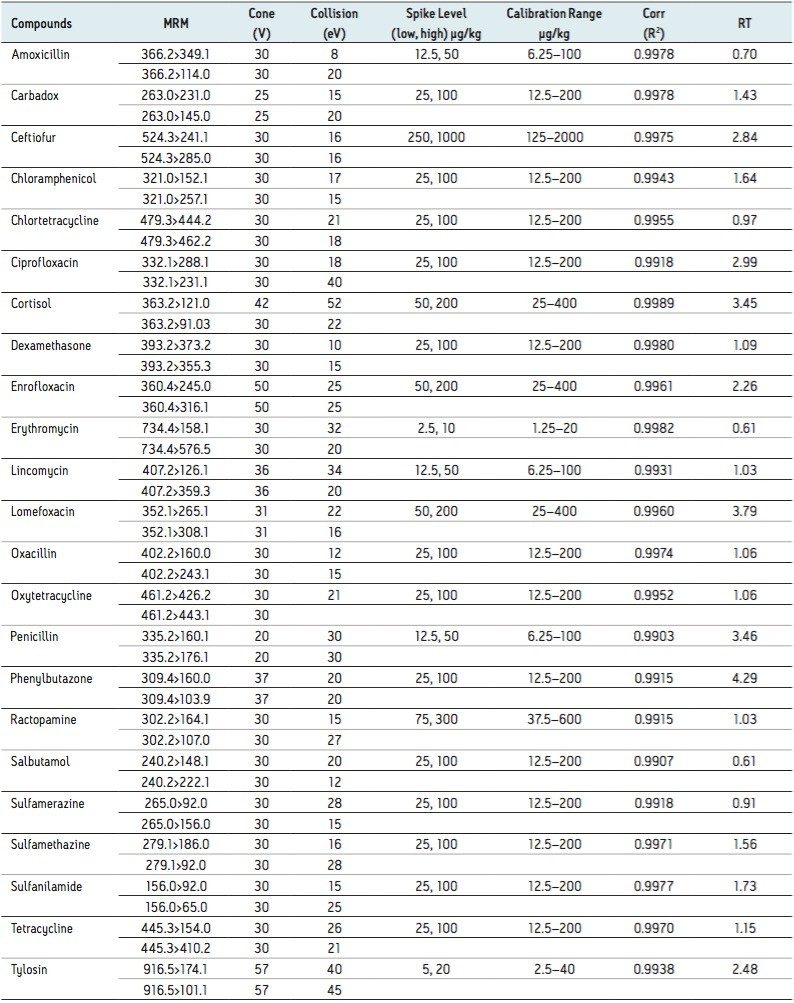
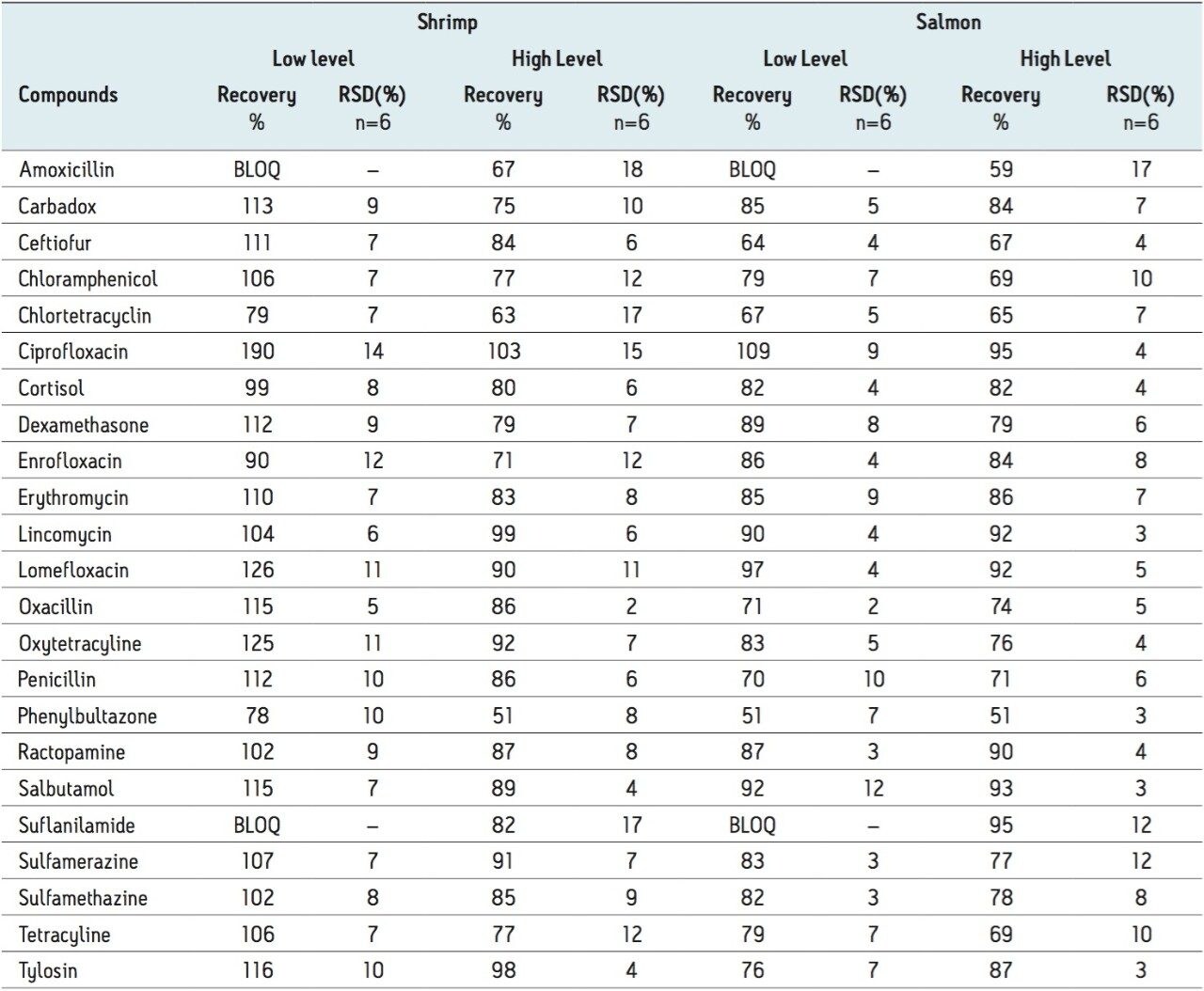
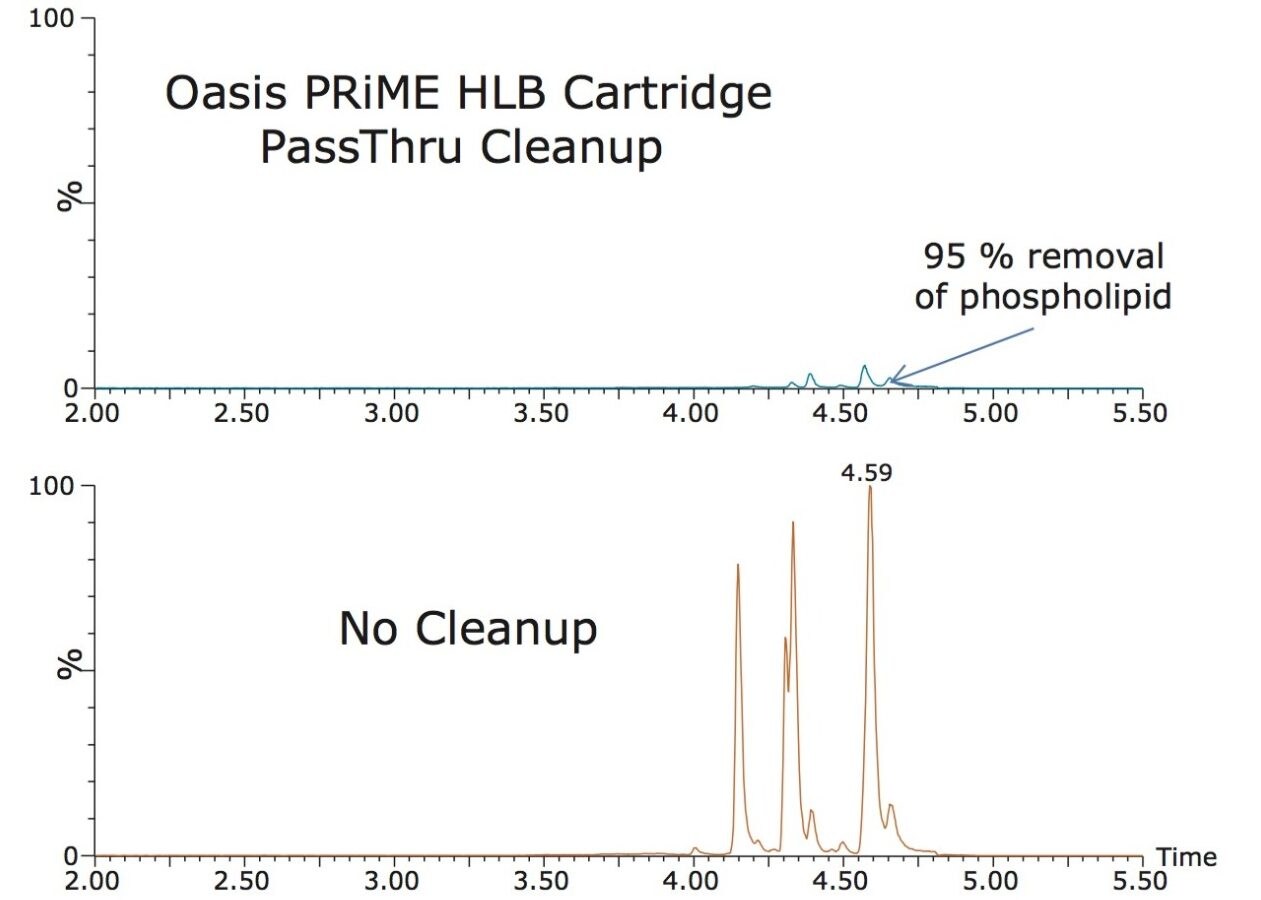
Table 2 shows the recovery data obtained from replicate analysis of spiked tissue samples. Matrix effects averaged about 40% for both shrimp and salmon. The chromatograms shown in Figure 1 show the effectiveness of the Oasis PRiME HLB Cartridge for removal of ≥95% of phopholipids from the shrimp extracts. The cartridge also removes more than 90% of hexane extractable fat.1
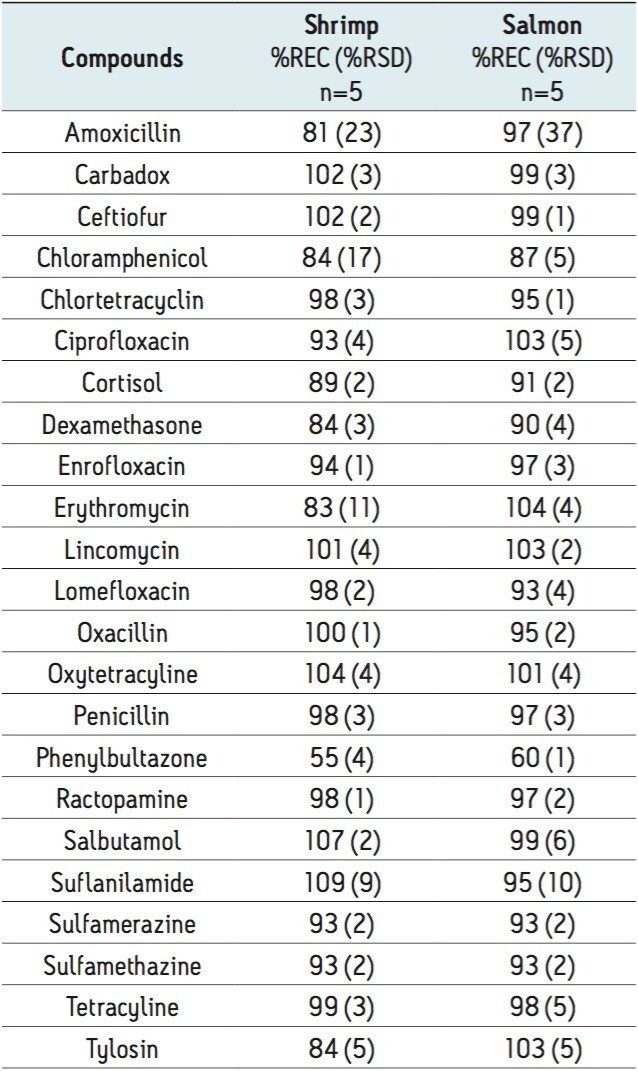
The procedure utilized in this study was developed from methods presented by Lehotay2 and refined by Tran.3 The overall method recoveries are generally above 70% but significantly lower recovery was observed for some of the more polar compound classes, such as tetracyclines. However, the Oasis PRiME HLB Cartridge cleanup contributes very little to any method recovery losses. As shown in Table 3, the measured recovery for the SPE cleanup step, specifically, is better than 80% in shrimp and better than 90% in salmon for all analytes except phenylbutazone.
A simple and effective extraction/protein precipitation procedure was applied to the analysis of shrimp and salmon tissue
A simple one-step pass-thru cleanup protocol using Oasis PRiME HLB Cartridges was employed to remove greater than 90% of fats and phospholipids from the initial extracts
The sample preparation methodology produced an extract that was free of particulates and required no subsequent filtration prior to LC-MS analysis
High and consistent recoveries were observed for a wide range of veterinary drugs using the simple one-step pass-thru cleanup protocol with Oasis PRiME HLB Cartridges
720005488, September 2015