
This application note demonstrates the application of the UPLC Amino Acid Analysis Solution to the Analysis of Animal Feed Hydrolysates to assure the nutritional requirements of the animals.
Waters UPLC Amino Acid Analysis Solution can provide accurate, reliable, and timely information. The robustness of the chromatography is shown by retention time stability of the standard across multiple columns, eluents, and days of analysis. The complexity of the samples does not distort retention time or other chromatographic features.
There are many factors that contribute to the health and growth of agricultural animals. Monitoring those factors is an important part to optimizing that growth, and therefore increasing profitability. Determination of the total amount of amino acids in the feeds provides critical information about the nutritional content. Based on this information, feeds can then be blended in an economical way while still assuring that the nutritional requirements of the animals are met.
The analysis of amino acids in animal feed samples is difficult due to the complex nature of the samples. The presence of a high concentration of non-protein constituents, including minerals, can interfere with the derivatization of amino acids and their subsequent analyses. These interferences can result in misidentification of the amino acids due to changes in retention over a series of analyses. Derivatization and chromatographic errors stemming from interferences will also result in incorrect quantitation of the amino acids. Unreliable amino acid analysis can lead to the costly and time consuming need to reanalyze samples. In the worst case, production may become uneconomical, and the animals may even become endangered.
Waters has developed the UPLC Amino Acid Analysis Solution as a turnkey solution for the analysis of amino acids in a variety of applications. By combining the well-established pre-column derivatization chemistry of AccQ•Tag (6-aminoquinolyl-Nhydroxysuccinimidyl carbamate) with the sensitivity and resolution of UltraPerformance LC (UPLC), this method consistently gives the right answer, even for these complex feed samples. Because the eluents, derivatization chemistry, and columns are application-tested, there is no need for method adjustments. A CD containing software projects for the management software (Empower) chromatography data system is supplied as part of the system. Results are quickly generated and reported.
The reliability of the application solution was tested in a collaborative study across multiple laboratories (the interlaboratory results will be reported elsewhere). Four animal feed hydrolysates, two soybean samples and two complex animal feed blends, were analyzed. The robustness and reproducibility of the method were evaluated in multiple experiments that included the typical sources of variability.
The complete sample set was analyzed on five separate days. Each daily experiment included fresh eluent and sample preparations, as well as five replicate derivatizations, injected in triplicate for each sample. A total of 75 data points were generated for each sample type. The ruggedness of the method was further tested by the inclusion of multiple columns throughout the five days of analysis.
Swine diet, poultry diet, whole soybean, and soybean meal samples were acid-hydrolyzed in an independent laboratory as part of a collaborative study. The samples were supplied at an estimated concentration of 1.0 mg/mL in 0.1 M HCl and sealed under argon in ampoules. Samples were stored at -80 °C until analysis. The standard was NIST 2389 Amino Acids in 0.1 mol/L HCl Reference Material, and it was diluted to 5, 100, and 250 pmol/μL.
The samples were diluted 1:16 with 0.1 M HCl prior to derivatization. The standard derivatization protocol was modified to include neutralization of excess acid with 0.1 M NaOH. The derivatization reagent reacts with both primary and secondary amines. The samples were derivatized in batches, and are stable at room temperature for up to one week when tightly capped. Conditions for pre-column derivatization and analysis are described in detail in the Waters UPLC Amino Acid Analysis Solution System Guide (P/N 71500129702). These derivatization conditions were modified to include additional base.
1. 60 μL AccQ•Tag Ultra Borate Buffer
2. 10 μL diluted sample
3. 10 μL 0.1 N NaOH
4. 20 μL reconstituted AccQ•Tag Ultra Reagent
The Waters UPLC Amino Acid Analysis Application Solution is provided with a CD that contains all the Empower methods necessary for acquisition and processing of the samples, as well as reporting of the results. Details of the methods can be found in the Waters UPLC Amino Acid Analysis Solution System Guide.
|
LC System: |
Waters ACQUITY UPLC System |
|
Column: |
AccQ•Tag Ultra, 2.1 x 100 mm, 1.7 µm (two columns were alternated) Part Number: 186003837 |
|
Column Temp: |
55 °C |
|
Sample Temp: |
20 °C |
|
Flow Rate: |
700 µL/min. |
|
Mobile Phase A: |
1:20 Dilution of AccQ•Tag Ultra Eluent A with MilliQ water (prepared fresh daily) Part Number: 186003838 |
|
Mobile Phase B: |
AccQ•Tag Ultra Eluent B Part Number: 186003839 |
|
Weak Needle Wash: |
95:5 Water:Acetonitirile |
|
Strong Needle Wash: |
5:95 Water:Acetonitrile |
|
Gradient: |
AccQ•Tag Ultra Hydrolysate Method (provided in the UPLC Amino Acid Analysis Solution) |
|
Total run time: |
9.5 min |
|
Injection volume: |
1 µL, Partial Loop with Needle Overfill |
|
Detection: |
UV (TUV), 260nm |
The contributions of all method factors to the variability of retention time of the NIST standard is summarized in Table 1. The reported value is the mean of fifteen injections, three injections on each of five days. The preparation of fresh mobile phases and the use of different columns throughout the five days did not contribute any instability to the retention time. The most variable amino acid, Histidine, shows a range of four seconds between the shortest and longest retention times.
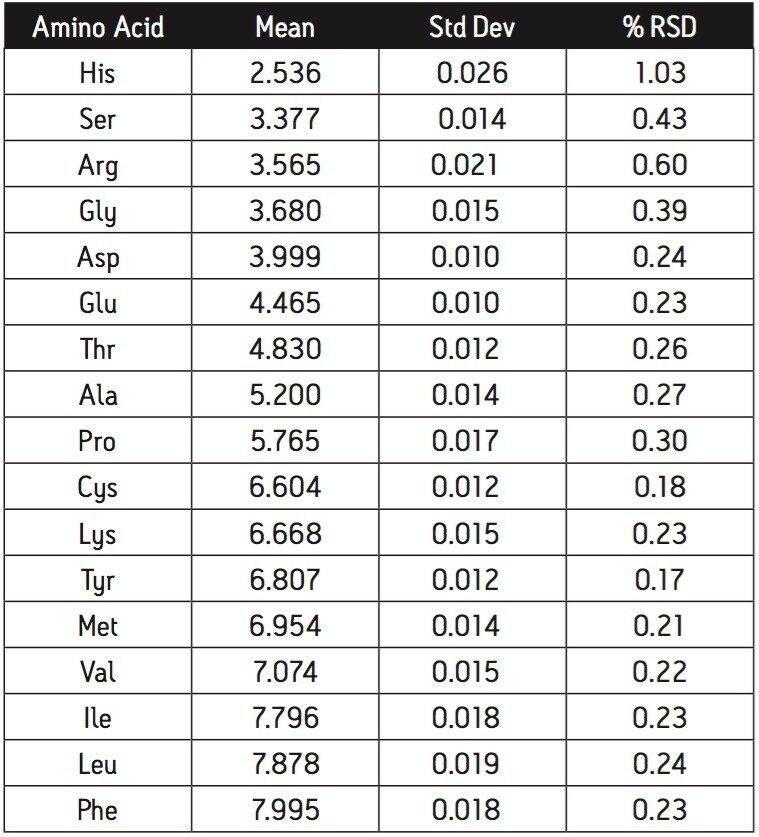
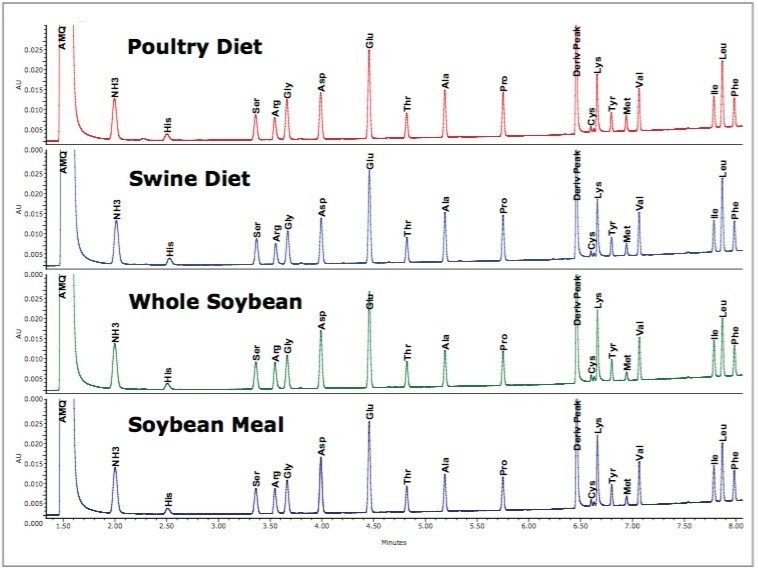
Figure 2 shows the separation of each of the different animal feed samples. The increased complexity of the diet samples does not result in any additional extraneous peaks or shifts in retention time that would result in the incorrect identification or quantitation of the amino acids.
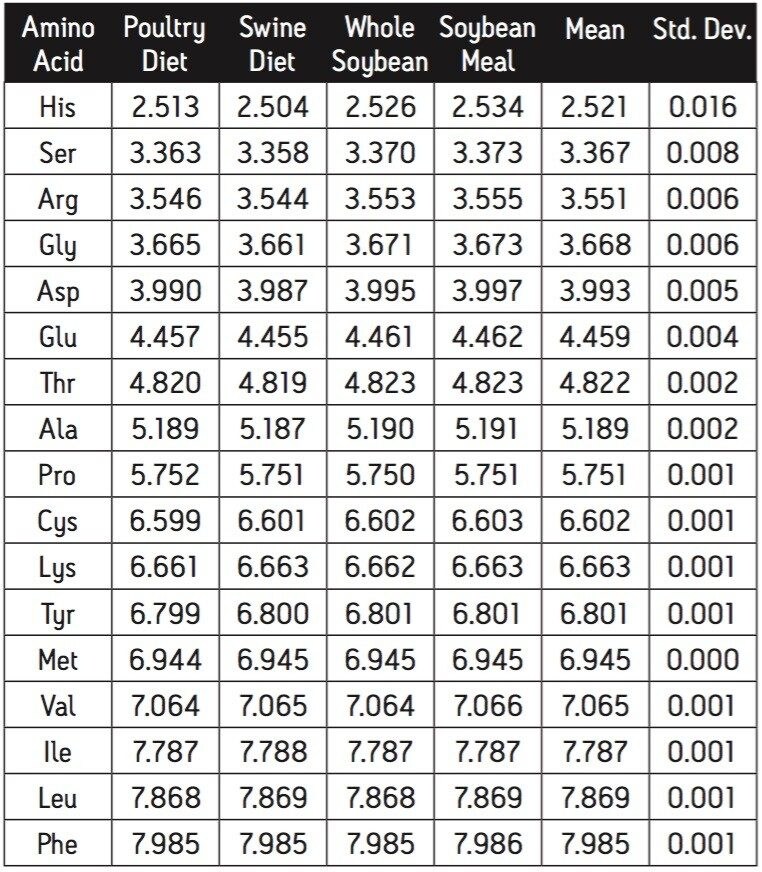
The retention times of the amino acids in the four different feed samples are shown in Table 2. The reported retention time is the mean for the fifteen analyses of that sample on a single day. The amino acids have the same retention time, independent of sample type. The greatest deviation, again for Histidine, is approximately two seconds.
The quantitative reproducibility, expressed as mole %, is shown in Table 3. For all four sample types tested across the five days of analysis, the mole % is typically better than 1% RSD. The sulfur-containing amino acids were not protected during hydrolysis, and are higher.
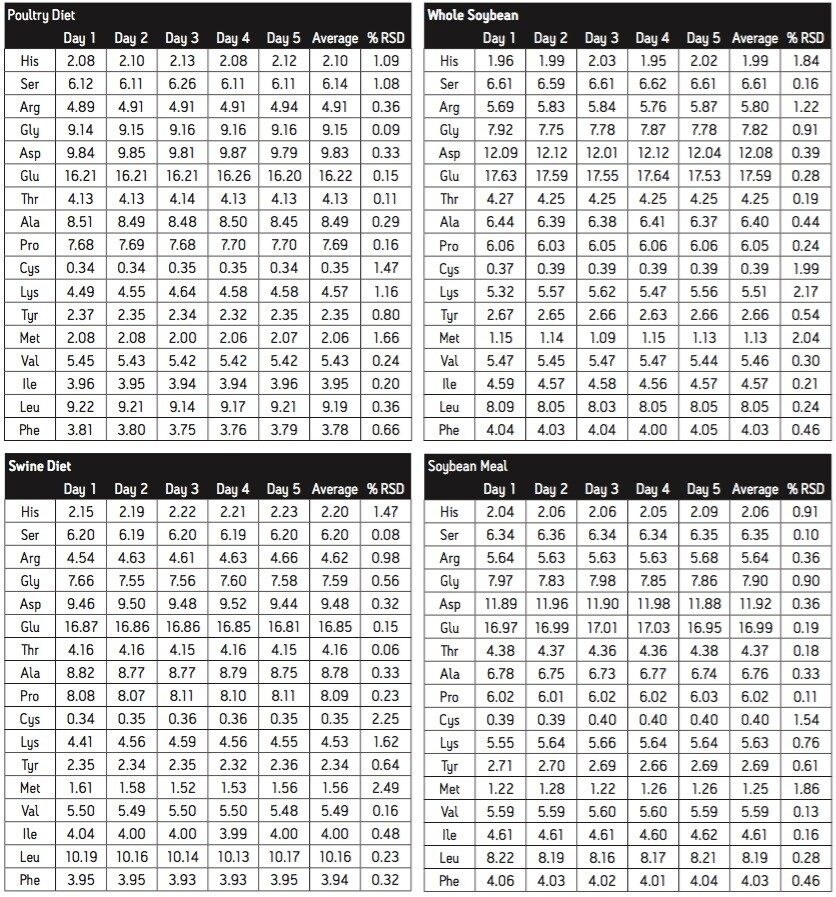
The composition of the four feed samples is, in general, similar. There are, however, clear distinctions. For example, Aspartate is lower in the blended feeds than in the soybean samples. Valine, on the other hand, is almost identical.
These 75 determinations reflect many sources of variability, including pipetting errors, incomplete derivatization, injector error, integration error, standard and sample preparation error, sample hydrolysis, and coelution of unidentified sample components with the amino acids. Complete examination of the raw data for each of the individual injections shows that the largest contribution to the variability is pipetting. The addition of an internal standard to the sample to be hydrolyzed will improve reliability. Norvaline is the preferred internal standard for this purpose.
The measurement of the total amount of amino acids plays a key role in the efforts to assess the quality and appropriateness of animal feeds for the best growth and production. While there are many methods available to obtain that information, the complexity of these kinds of samples can compromise the reliability of the results. In that case, samples might require reanalysis, or, in the worst case, the most economical growth conditions can be misjudged.
The summary of this study of animal feed hydrolysates demonstrates that the Waters UPLC Amino Acid Analysis Solution can provide accurate, reliable, and timely information. The robustness of the chromatography is shown by retention time stability of the standard across multiple columns, eluents, and days of analysis. The complexity of the samples does not distort retention time or other chromatographic features.
Quantitation is reliable and reproducible. The sensitivity of the analytical method requires only small aliquots of these complex feed samples.
These experiments show that the Waters UPLC Amino Acid Analysis Solution can be used to determine the nutritional content of animal feeds. The turnkey solution provides the derivatization chemistry, the chromatographic column and eluents, and the software for data acquisition and reduction so that the end user can be assured accurate and precise results.
720002804, September 2008