
This application note demonstrates the detection of additives in polymer mixtures employing reversed phase chromatography in conjunction with evaporative light scattering detection.
Health care formulations, personal care products and industrial processing mixtures contain polymers and additives; the latter ones are usually present between 0.1–3%. These additives are low molecular weight compounds that, while present in relatively low concentrations, play an important role. For instance, they can act as light stabilizers or antioxidants in hair sprays and gels. Other additives play the role of flame retardant; and, in industrial processes, these compounds become essential as plasticizers and slip agents.
Polymer characterization and additive separation are commonly carried out employing refractive index-based chromatographic systems and refractive index detectors respond well under isocratic conditions. A significant number of HPLC users in the pharmaceutical, cosmetic and industrial sectors find themselves looking for techniques that will increase their throughput in the lab. Evaporative light scattering is the answer because this technique allows scientists to carry out shorter runs under gradient conditions. Some additives lack chromophores, therefore they are UV/Vis transparent and evaporative light scattering becomes an attractive alternative technique.
In this work we discuss the detection of additives in polymer mixtures employing reversed phase chromatography in conjunction with evaporative light scattering detection.
A Waters Alliance System with a column oven, a 2996 UV/Vis and a 2420 Evaporative Light Scattering Detector (ELSD) were employed in this application. A Waters Symmetry C18 Column, 5 µm, 3 x 150 mm was employed in this work.
A linear gradient from 70% to 100% B in 10 minutes was used at a flow rate of 0.6 mL/min and column temperature set at 60 °C. The mobile phases were as follows: A: DIWater and B: Acetonitrile. The ELSD parameters were set at: Nebulizer 70% power, drift tube temperature of 40 °C and nebulizer pressure of 40 psi.
The sample used in this work was a mixture of stabilizers, antioxidants and slip agents. In particular, the sample was composed of the following chemicals: Tinuvin P (0.23 mg/mL), BHT (0.23 mg/mL), Luwinox (0.17 mg/mL), Succonox 18 (0.18 mg/mL), Naugard 445 (0.21 mg/mL), Tinuvin 328 (0.20 mg/mL), Irganox 1010 (0.19 mg/mL), Irganox 1330 (0.21 mg/mL), Irgafos 168 (0.23 mg/mL), Irganox 1076 (0.21 mg/mL), Crodamide (0.16 mg/mL), and Tinuvin 312 (0.20 mg/mL).
An ELSD has three sections as depicted in Figure 1 - a nebulization section where the column eluent comes in contact with the vaporization gas forming an aerosol, a desolvation section where the a e rosol droplets are heated and the solvent evaporates leaving dry particles and finally a detection section where particle scatter is mesured. The formation of aerosol droplets happens in the nebulizer, the desolvation occurs in the drift tube and the scatter is measured in the scatter chamber. All areas are heated, the nebulizer and drift tube can be controlled by the user, but the scatter chamber is kept at constant temperature to avoid product performance issues due to sample deposition in the chamber.
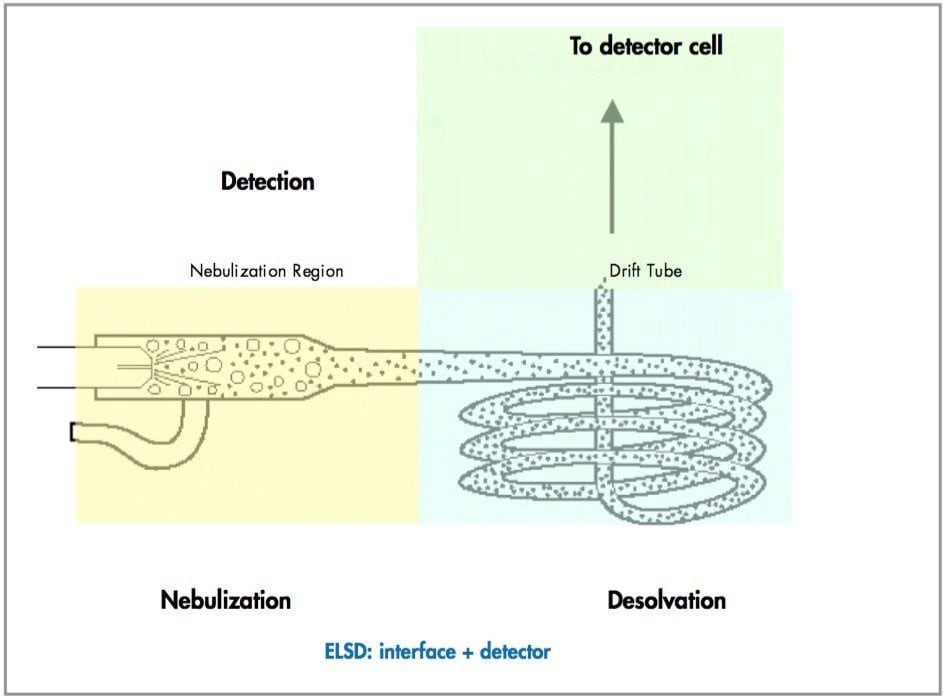
Nebulization
Desolvation
Detection
Figure 2 shows the chromatographic results when injecting the mixture of compounds detailed in the Experimental section. As seen in the figure, a fast reversed-phase chromatographic method was successfully developed for the analysis for polymer additives using a C18 column. Evaluating the results presented in Figure 2, it was noticed that Crodamide, a slip agent used in a variety of applications including the food industry, was not detected at 220 nm, but had a significant response under ELS detection. Also, Naugard 445 has a more important peak in ELSD in contrast to UV. Further BHT is detected by UV and not by ELSD. This lack of detection in ELS mode is the result of BHT physical characteristics. These results show that response variations between UV and ELSD can be significant and that the new Waters 2420 provides good sensitivity for non-UV absorbing compounds. However, as seen in Figure 2, not all compounds can be seen by all detectors. In fact, the results presented here indicate that employing more than one detector in a chromatographic system can aid in the detection of compounds varying in nature within the same sample decreasing the number of injections needed to characterize more completely and effectively a sample.
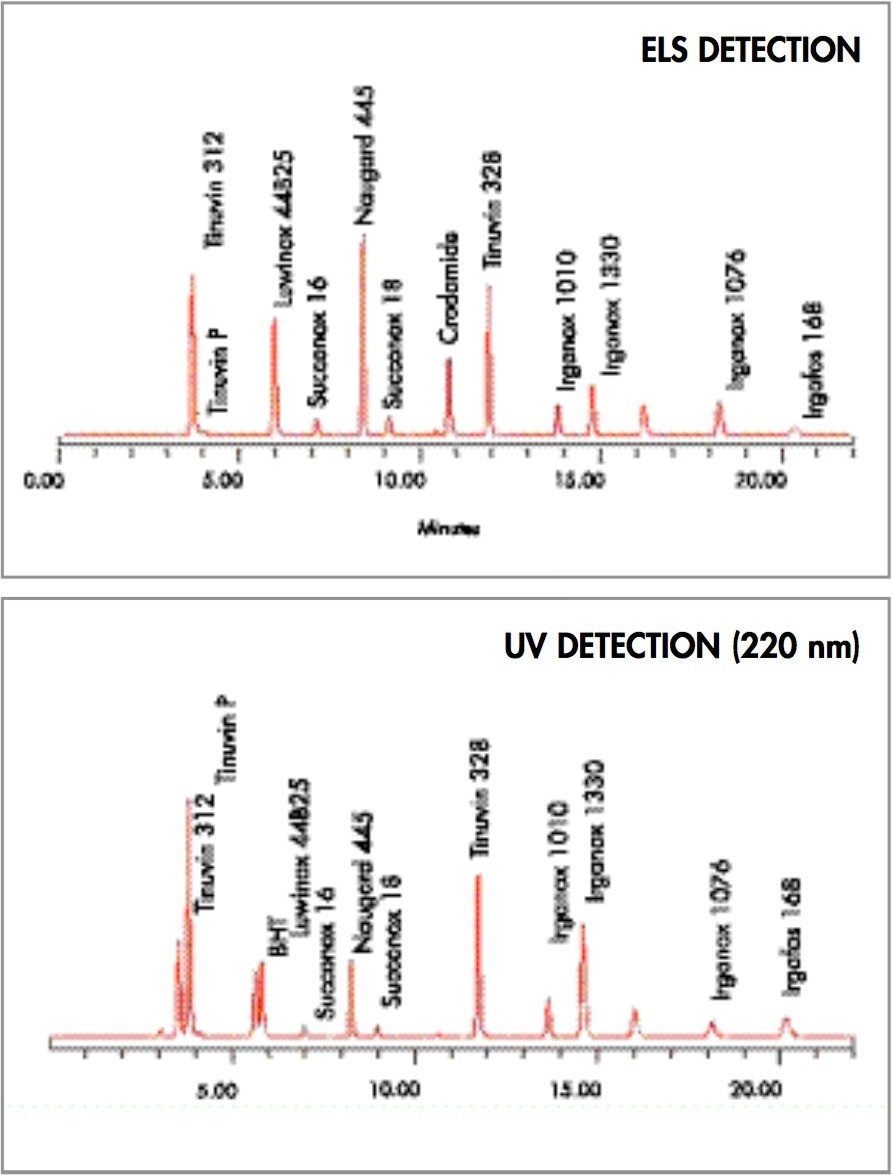
A fast LC method based on reversed-phase chromatography was used to separate a mixture of polymer additives. The Waters 2420 ELSD was successfully used in an LC/UV system for the detection of slip agents. In fact, the 2420 1 ELSD presented good sensitivity for crodamide, a widely employed slip agent. The results presented here indicate that not all compounds can be seen by all detectors and that by employing complementary detectors it is possible to obtain more information per chromatographic run increasing lab productivity.
720000959, August 2004