Analysis of Amikacin and Kanamycin in Pharmaceutical Products with 3465 Electrochemical Detector
Abstract
Amikacin (AMI) and kanamycin (KAN) were analyzed by HPAEC-PAD according to the United States Pharmacopoeia (USP) Monographs. The instrument setup included an ACQUITY™ Arc™ System and a 3465 Electrochemical Detector (ECD). The instrument control and data acquisition were handled by an Empower™ 3 Chromatography Data Software (CDS). The analytical performance met the USP system suitability requirements for both analytes in resolution, tailing factor, and repeatability. The 3465 ECD demonstrated an excellent linear response in concentration ranges that are sufficient for the assay of AMI and KAN. Amikacin injection samples were analyzed using this setup with satisfactory results, demonstrating the suitability of the ACQUITY Arc System and the 3465 ECD detector for the assay of amikacin and kanamycin in pharmaceutical products.
Benefits
- ACQUITY Arc with 3465 ECD provides reliable analysis of amikacin and kanamycin, meeting the USP performance requirements
- 3465 ECD demonstrates an excellent linear response in concentration ranges sufficient for the assay of amikacin and kanamycin
Introduction
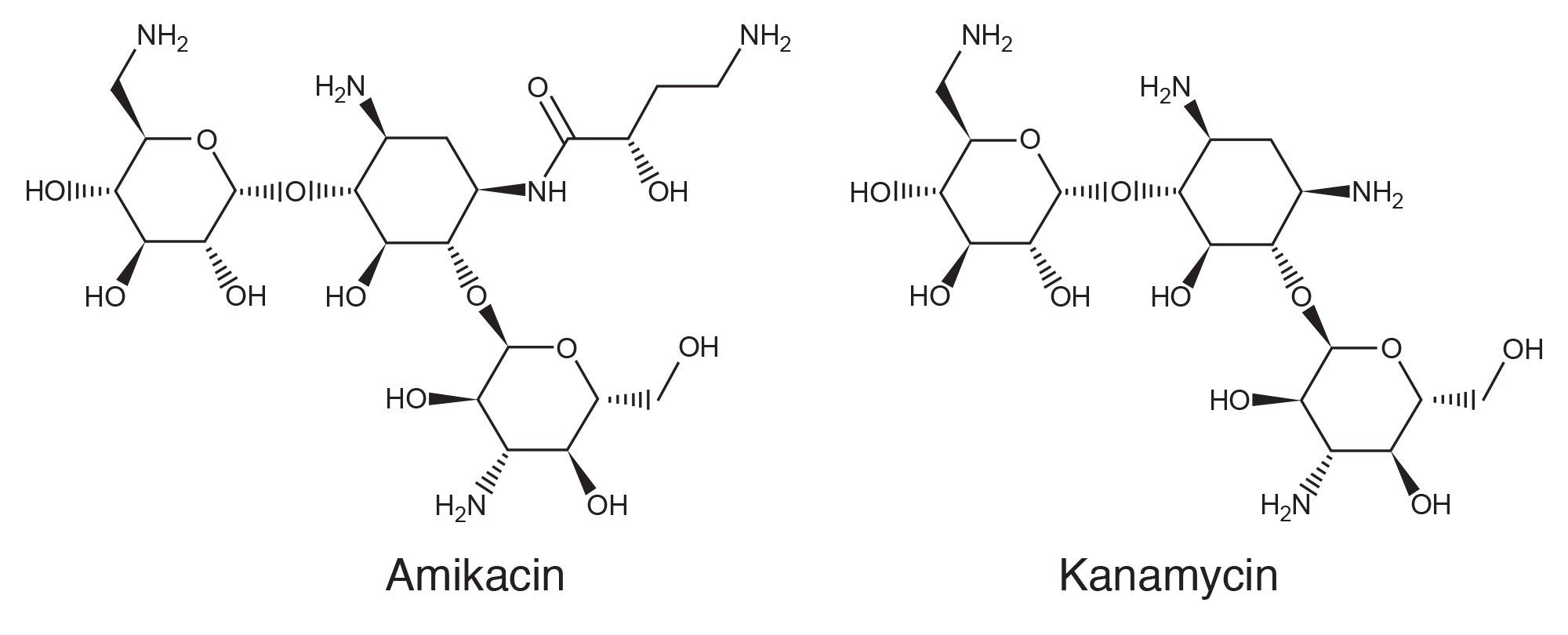
Amikacin and kanamycin are closely related aminoglycoside antibiotics and are often used for treatment of bacterial infections and tuberculosis. KAN is isolated from the bacterium Streptomyces kanamyceticus. AMI is derived from KAN by the acylation of an amino group of KAN. Figure 1 shows the structures of AMI and KAN. The USP has issued AMI and KAN monographs in which High Performance Anion Exchange Chromatography (HPAEC) with Pulsed Amperometric Detection (PAD) was used for the assay.1–5 This application note describes the HPAEC-PAD assay of AMI and KAN on an ACQUITY Arc System with a 3465 ECD, demonstrating its performance and suitability for the analysis of AMI and KAN in pharmaceutical products.
Experimental
Standards and Reagents
Kanamycin sulfate and amikacin (CRM) were purchased from Sigma-Aldrich, Inc. (St. Louis, MO). Amikacin Injections (250 mg/mL) were obtained from a local provider. Low-carbonate sodium hydroxide (NaOH) solution (50% w/w Certified, Fisher Chemical™, carbonate ≤0.10%) was purchased from Fisher Scientific Co. (Pittsburgh, PA). Methanol (for HPLC) was purchased from Honeywell Research Chemicals (Charlotte, NC). Deionized water (>18.2 Mohm∙cm) was generated in-house.
Preparation
Stock standard solutions of KAN and AMI were prepared by dissolving standards in deionized water at 10 mg/mL. A standard mix solution of KAN and AMI at 0.008 mg/mL and 0.020 mg/mL, respectively, were prepared from the stock solutions.
AMI Injection sample solutions were prepared by dilution with deionized water to 0.020 mg/mL AMI (based on the label concentration).
Mobile phases of 115 mM and 134 mM NaOH were prepared by mixing of certain amount of low-carbonate 50% w/w NaOH solution with deionized water to make a 1 liter NaOH solution. The deionized water was degassed prior to be used in the mobile phase preparation. Fresh mobile phase was prepared daily.
HPAEC-PAD Conditions
|
System: |
ACQUITY Arc System (QSM-R and SM FTN-R) with a 3465 ECD |
|
Software: |
Empower 3 (3.6.1) Chromatography Data Software |
|
Column: |
Hamilton® RCX-10 Analytical Column 250 x 4.6 mm I.D. 7 µm (PEEK) with a RCX-10 guard column |
|
Vial: |
PP vial 700 µL volume with cap and preslit septum (p/n: 186005221) |
|
Temp.: |
30 °C for column and detection flow cell |
|
Flow cell: |
FlexCell™ with a gold working electrode, a HyREF reference electrode and a stainless-steel auxiliary electrode, 50 µm spacer (p/n: 700013068). |
|
Mobile phase: |
115 or 134 mM NaOH solution (protected under N2 gas using a solvent stabilization kit (p/n: 205001099) |
|
SM purge/wash solvent: |
Deionized water |
|
Seal wash solvent: |
Water:methanol 8:2 (v/v) |
|
Flow rate: |
0.5 mL/min |
|
Run time: |
30 min |
|
Injection volume: |
10 µL |
|
Injector loop offline: |
0.5 min |
|
Detection: |
PAD |
|
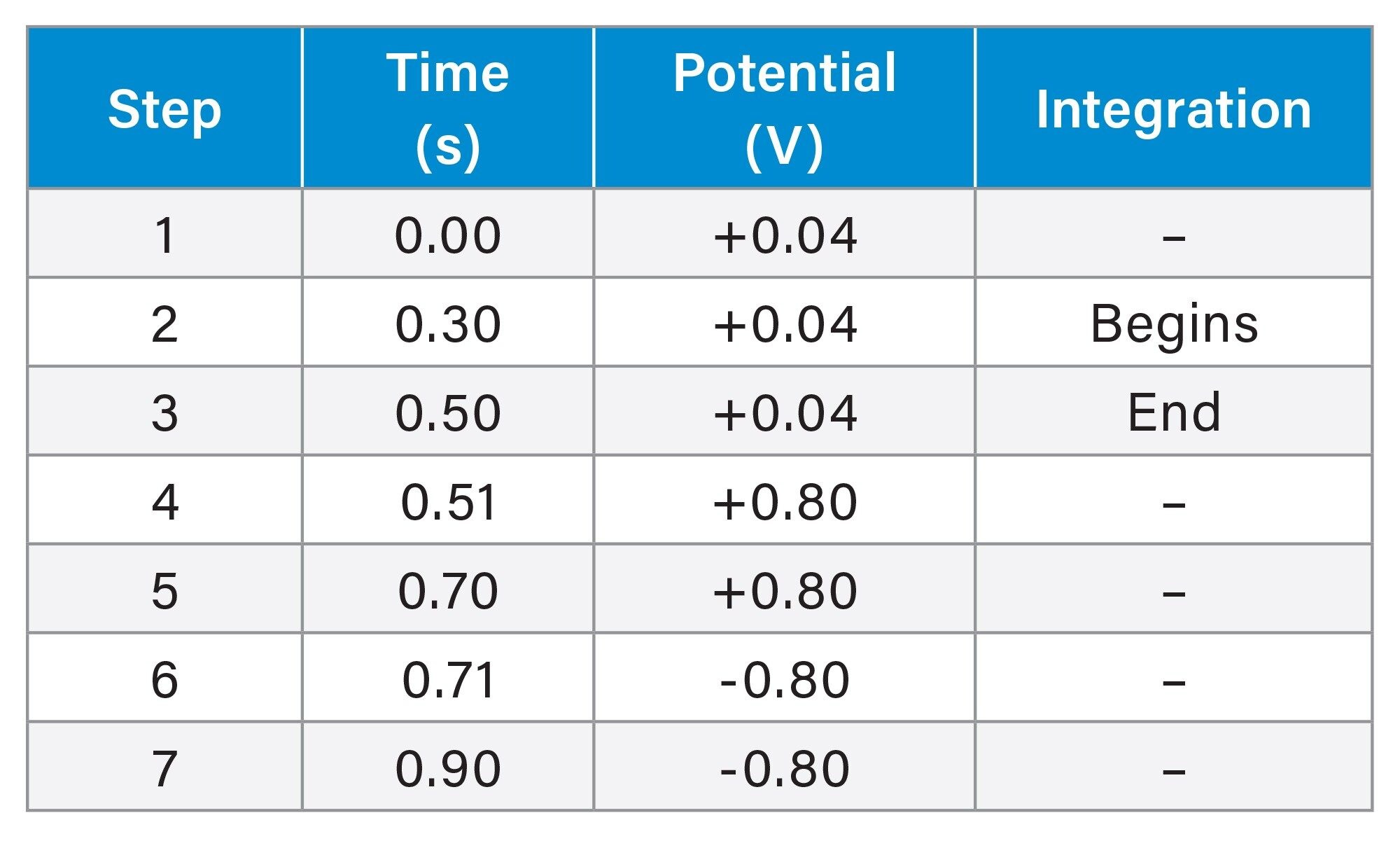
Potential waveform: |
See Table 1 |
|
ADF: |
0.02 Hz |
Working with High pH Mobile Phases
Additional precautions were taken in working with these mobile phases (pH up to 13.1). The precaution steps include a) PEEK tubing (0.004 inch i.d.) was used to connect the injector valve to the columns and further to the detector flow cell; b) the injector loop was taken offline after injection (at 0.5 minute); and c) deionized water was used to flush the system and columns after daily use. There was no leakage or carry-over observed in the study (about 500 injections of standards and samples).
Results and Discussion
Detector Flow Cell
The detector flow cell consisting of a gold working electrode, a hydrogen reference electrode (HyREF), and an auxiliary stainless-steel electrode was used in the analysis. The USP Monographs recommend a flow cell with a gold working electrode and a silver-silver chloride reference electrode. The silver-silver chloride reference electrode requires regular maintenance while the HyREF does not. The auxiliary electrode helps to improve the dynamic range of detection. Based on these considerations, the FlexCell with a gold working electrode, a HyREF reference electrode, and a stainless-steel auxiliary electrode was used.
System Suitability Test
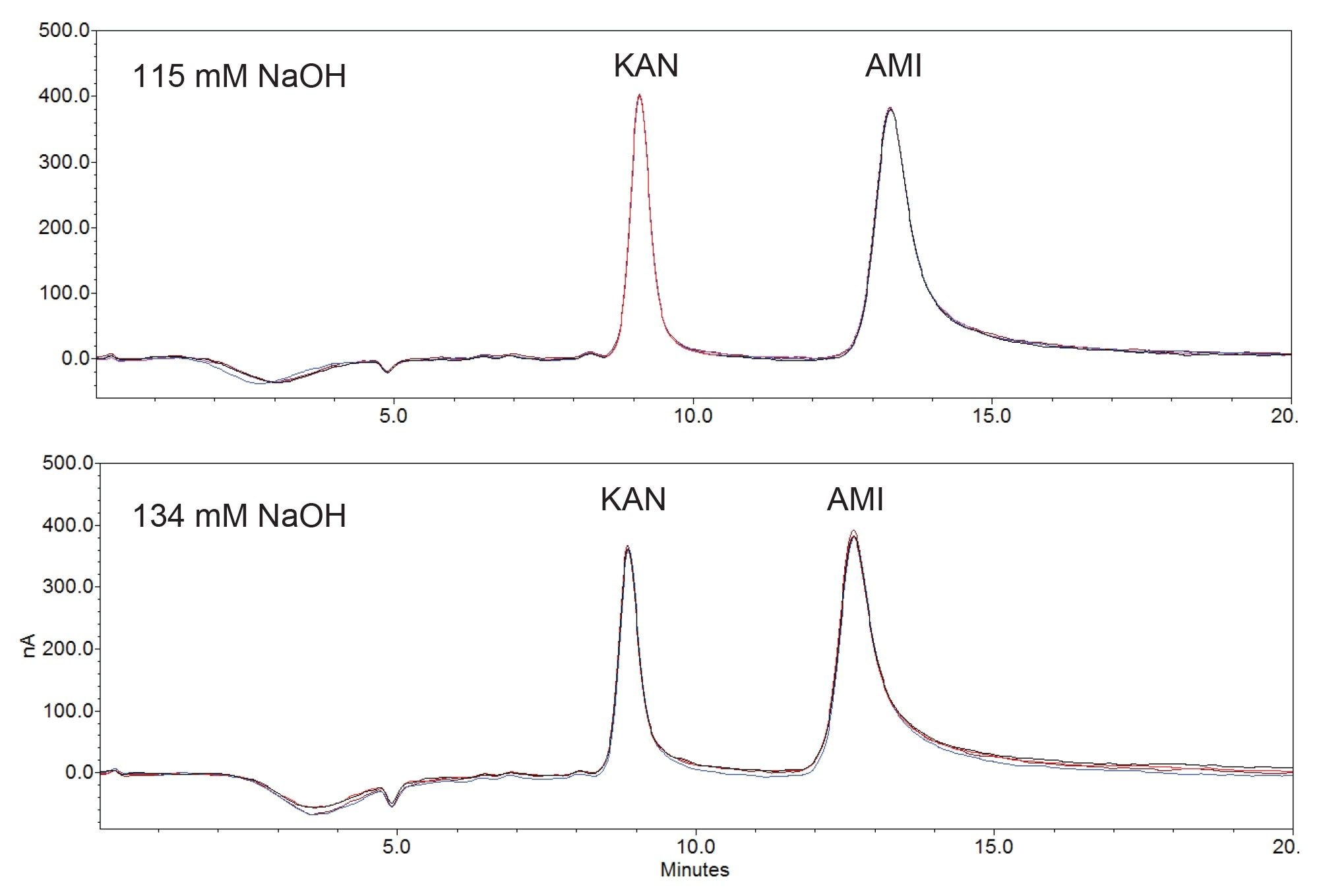
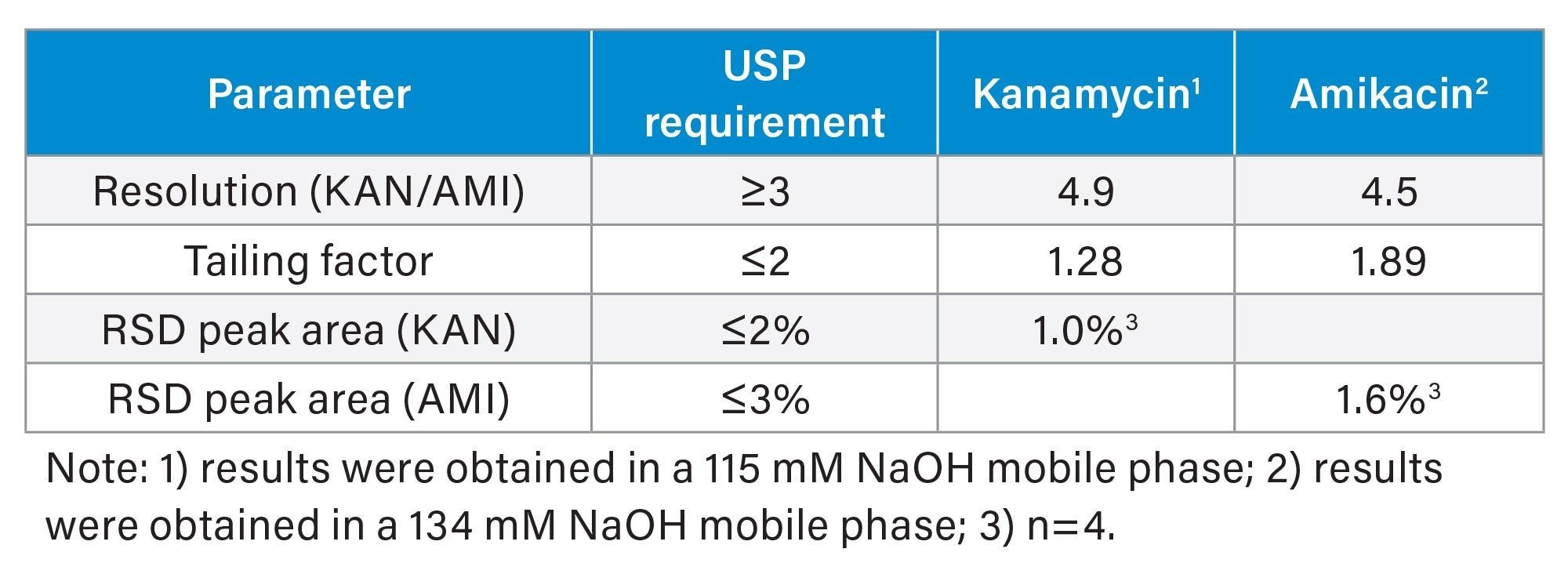
USP Monographs for AMI and KAN specify a set of performance criteria for the assay.1–5 The assay conditions for both analytes are the same except for the mobile phase. For KAN a mobile phase of 115 mM NaOH solution is recommended and for AMI a 134 mM NaOH solution is recommended. Table 2 shows the USP criteria and the measured results using a standard mix solution of 0.008 mg/mL KAN sulfate and 0.020 mg/mL AMI under the specified mobile phase conditions. Figure 2 shows an overlay of the chromatograms obtained from the standard mix solution in USP specified mobile phases. These results meet all USP system suitability requirements (Table 2).
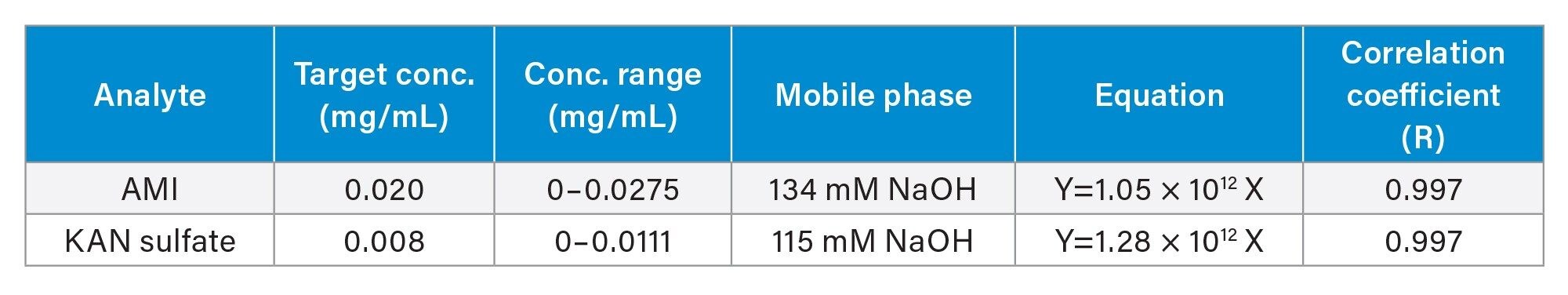
Linearity
USP Monographs for AMI and KAN specify single point (level) calibrations, which require a linear (linear through zero) relationship between the detector’s response and the concentration. The detector’s response linearity was investigated. Table 3 shows calibration results for AMI and KAN in concentration ranges up to 140% of the target concentrations in the USP specified mobile phases. Data points at six concentration levels were fitted by a least square regression to lines (through zero). The correlation coefficient values for both analytes were 0.997, demonstrating an excellent linear relationship between the peak area and the analyte concentration. An injection volume of 10 µL was used in this study.
Sample Analysis
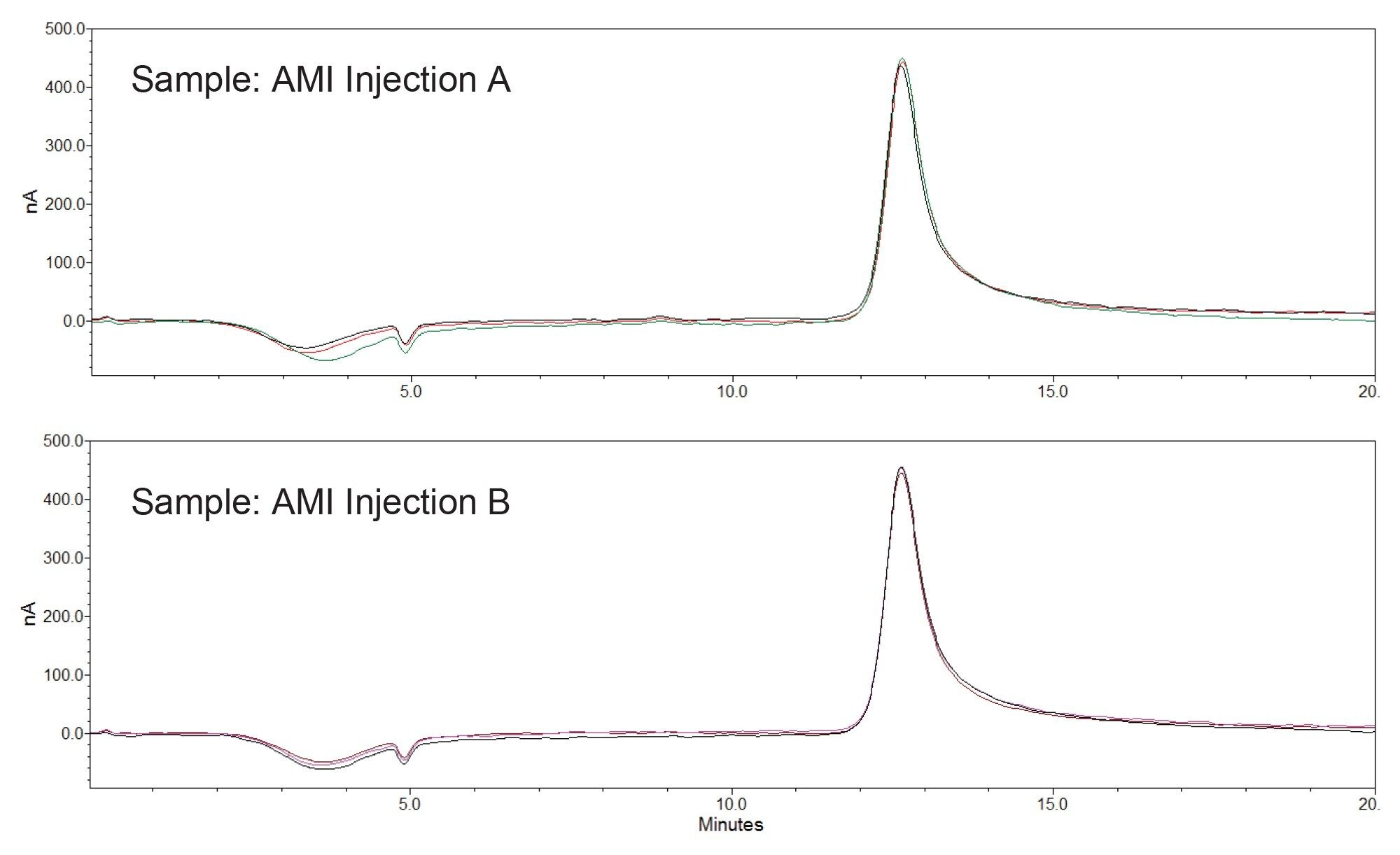
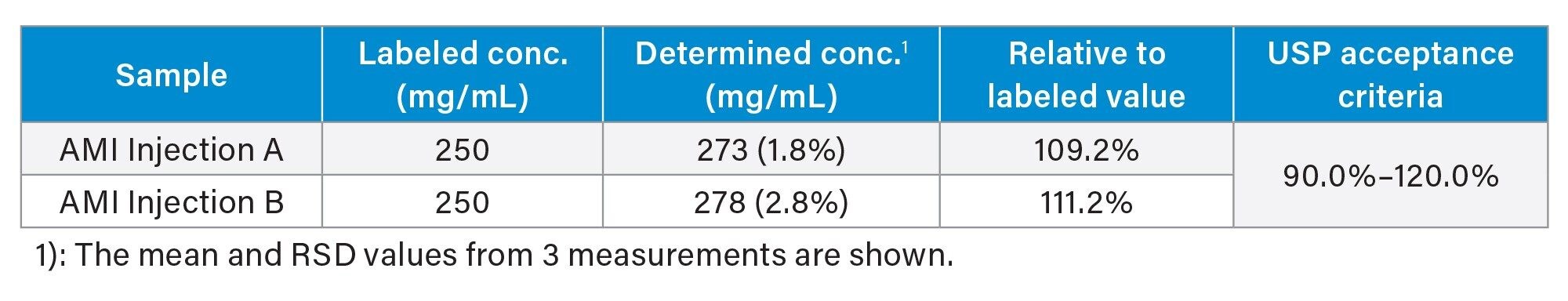
Table 4 shows AMI assay results for two AMI Injection samples. These determined concentration results are within the USP acceptance criteria for AMI (90.0%–120.0%). Figure 3 shows the chromatograms of triplicate injections of these samples.
Conclusion
This work demonstrates that the ACQUITY Arc System with a 3465 Electrochemical Detector under the Empower 3 CDS control provides a reliable and accurate assay of AMI and KAN in pharmaceutical products. The analytical performance meets the USP performance requirements for the peak resolution, tailing and reproducibility for AMI and KAN.
References
- United States Pharmacopeia (2022), USP Monographs. Kanamycin Injection, USP43-NF38 – 2501. Rockville, MD: United States Pharmacopeia.
- United States Pharmacopeia (2022), USP Monographs. Kanamycin Sulfate, USP43-NF38 – 2502. Rockville, MD: United States Pharmacopeia.
- United States Pharmacopeia (2022), USP Monographs. Amikacin, USP43-NF38 – 219. Rockville, MD: United States Pharmacopeia.
- United States Pharmacopeia (2022), USP Monographs. Amikacin Sulfate, USP43-NF38 – 220. Rockville, MD: United States Pharmacopeia.
- United States Pharmacopeia (2022), USP Monographs. Amikacin Sulfate Injection, USP43-NF38 – 221. Rockville, MD: United States Pharmacopeia.
720007647, May 2022