For research use only. Not for use in diagnostic procedures.
Here we describe a clinical research method using liquid-liquid extraction of plasma with 5-fluorouracil-13C15N2 internal standard.
5-fluorouracil is an anti-cancer agent which acts by interfering with the synthesis of deoxyribonucleic acid (DNA) and, to a lesser extent, inhibits the formation of ribonucleic acid (RNA). Since there is a high degree of inter-individual variability for its metabolism,1 an analytically sensitive and selective measurement procedure may aid in pharmacokinetic and pharmacodynamic clinical research studies.
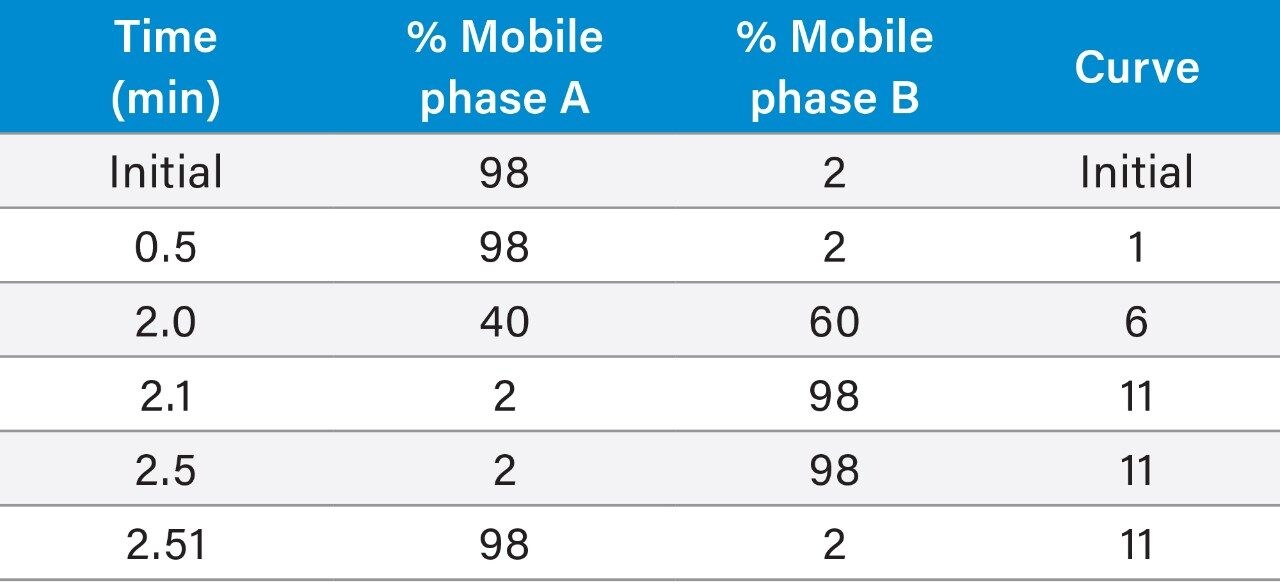
Here we describe a clinical research method using liquid-liquid extraction of plasma with 5-fluorouracil-13C15N2 internal standard. Chromatography was achieved within 3 minutes using a Waters ACQUITY UPLC HSS PFP Column (2.1 x 100 mm, 1.8 µm) on a ACQUITY UPLC I-Class System followed by detection on a Xevo TQD Mass Spectrometer (Figure 1).

Sample preparation: 5-fluorouracil certified reference standard and its stable labeled internal standard (13C15N2) were purchased from Toronto Research Chemicals (Toronto, Ontario, Canada). Calibrators were prepared in a surrogate matrix of pooled plasma purchased from Golden West Biologicals (Temecula, CA, USA). The calibration range for 5-fluorouracil was 20–2000 ng/mL. QC materials were prepared independently using this same pooled plasma at 40, 350, 750, and 1500 ng/mL.
Sample extraction: To 50 µL of sample, 5 µL of 10 µg/mL internal standard (ISTD) in methanol was added and then vortex mixed for 30 seconds. 500 µL of ethyl acetate containing 0.1% formic acid by volume was then added, then vortex mixed for 30 seconds and centrifuged for 2 minutes at 16,100 g. A 400 µL aliquot of supernatant was evaporated to dryness under nitrogen at 40 °C. The sample was reconstituted using 75 µL 0.1% formic acid in water prior to analysis on the UPLC-MS/MS system.
|
System: |
ACQUITY UPLC I-Class (FTN) |
|
Needle: |
30 μL |
|
Column: |
ACQUITY UPLC HSS PFP Column |
|
Mobile phase A: |
Water |
|
Mobile phase B: |
Acetonitrile |
|
Needle wash solvent: |
80% aqueous methanol + 0.1% formic acid |
|
Purge solvent: |
Mobile phase A |
|
Seal wash: |
20% aqueous methanol |
|
Column temp.: |
35 °C |
|
Injection volume: |
20 μL |
|
Flow rate: |
0.40 mL/min |
|
Run time: |
3.0 minutes (3.5 minutes injection to injection) |
|
System: |
Xevo TQD |
|
Resolution: |
MS1 (0.7 FWHM) MS2 (0.7 FWHM) |
|
Acquisition mode: |
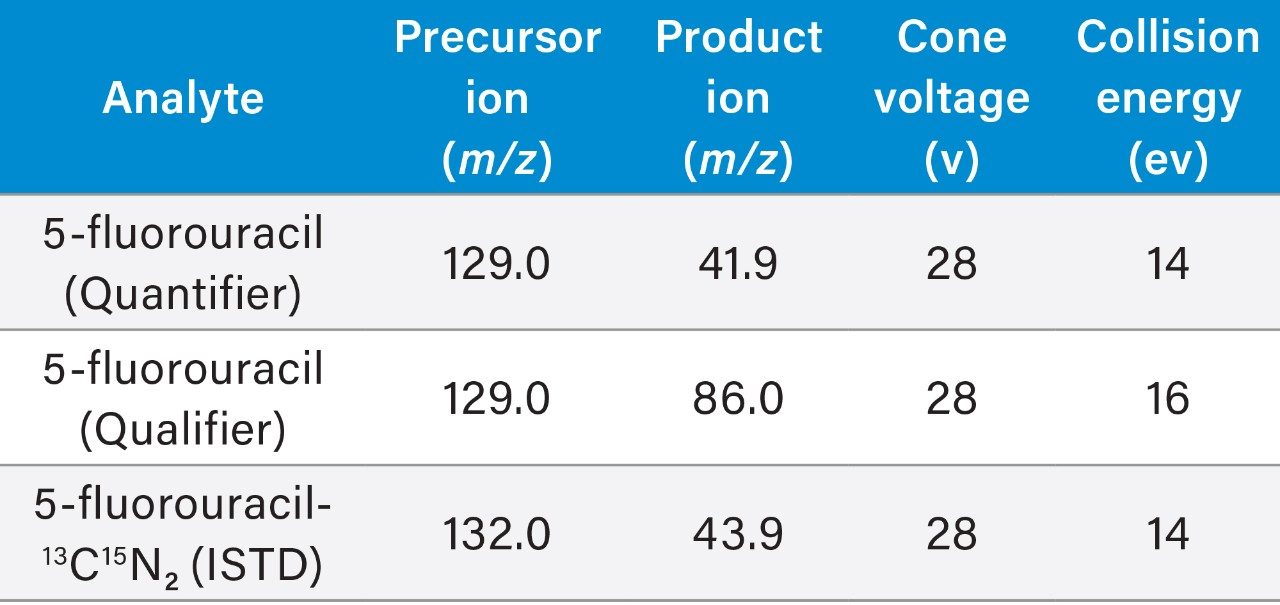
Multiple Reaction Monitoring (MRM) (See Table 2 for details) |
|
Polarity: |
ESI negative ionization |
|
Capillary: |
3.0 kV |
|
Source temp.: |
140°C |
|
Desolvation temp.: |
450 °C |
|
Dwell time: |
0.05 seconds (5-fluorouracil), 0.015 seconds (ISTD) |
|
Inter-scan delay: |
0.02 seconds |
|
Inter-channel delay: |
0.01 seconds |
MassLynx v4.1 Software with TargetLynx Application Manager
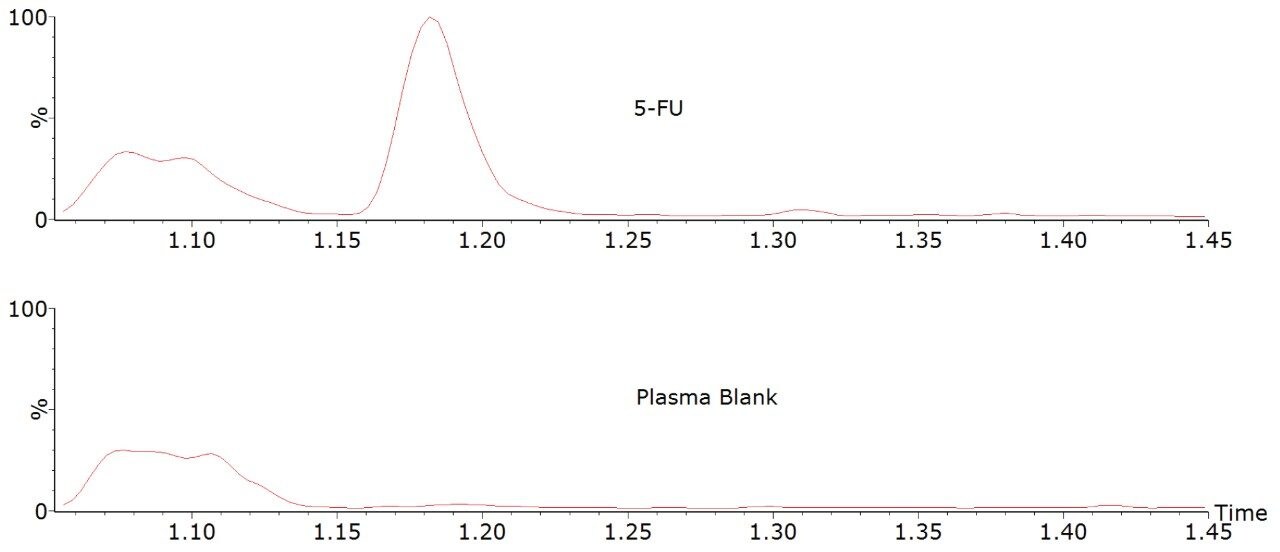
Under these chromatographic conditions, 5-fluorouracil is separated from an isobaric interference (at approximately 1.1 minutes) extracted from plasma. The retention time of 5-fluorouracil is approximately 1.18 minutes.
No system carryover was observed following analysis of plasma samples with 5-fluorouracil levels of up to 10,000 ng/mL.
Analytical sensitivity investigations indicate that quantification (<20% RSD, <15% bias) at 7.5 ng/mL for 5-fluorouracil is achievable.
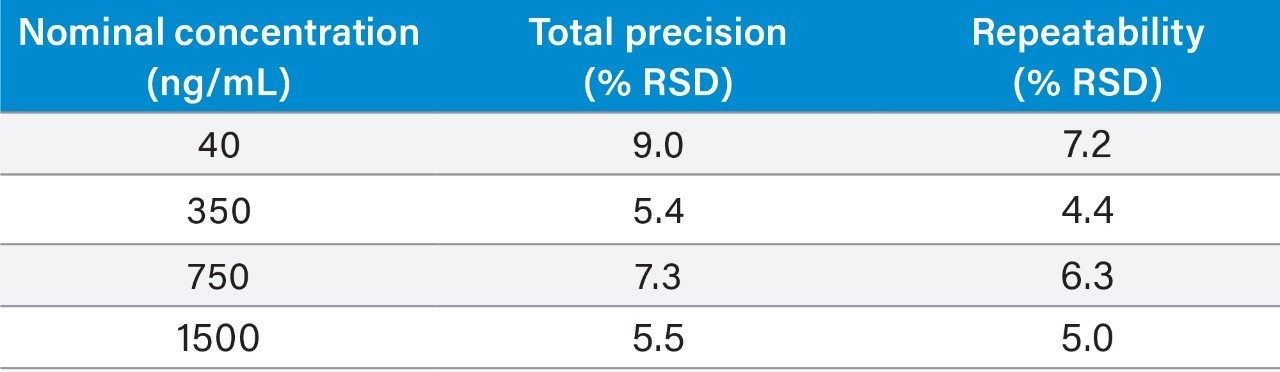
Precision experiments were performed, extracting and quantifying five replicates of four concentrations of QC material over 5 separate days (n=25). Repeatability was assessed by analysing five replicates at each QC level. Table 3 presents the results of these experiments, where total precision and repeatability at the four concentrations assessed was ≤9.0% RSD for 5-fluorouracil.
The method was shown to be linear over the range of 14–2600 ng/mL when different ratios of high and low concentration pools of 5-fluorouracil were combined and analysed.
Matrix effects were evaluated at low (40 ng/mL) and high (1500 ng/mL) 5-fluorouracil concentrations in plasma (n=6). The matrix factor range was 0.62 to 0.69 (mean 0.66) for low concentrations compared with 0.46 to 0.75 (mean 0.65) for high concentrations. Use of an isotopically labeled 5-fluorouracil internal standard compensated for the observed matrix effects yielding matrix factor ranges of 0.94 to 0.98 (mean 0.96) and 0.96 to 1.05 (mean 1.02) for low and high concentrations, respectively.
Potential interference from endogenous compounds (albumin, bilirubin, cholesterol, triglycerides, and uric acid) and the exogenous material intralipid (20% emulsion) spiked at high concentrations was assessed by determining the recovery of 5-fluorouracil (n=3) from low and high pooled plasma samples (40 ng/mL and 1500 ng/mL). Recovery ranged from 90.5–110.6%. When assessing the effects of the presence of acetaminophen, fluconazole, 5,6-dihydro-5-fluorouracil, ketoconazole, itraconazole, methotrexate, phenytoin, posaconazole, uracil, and voriconazole, recovery ranged from 91.8–108.0%. A substance was deemed to interfere if a recovery range of 85%–115% was exceeded.
External Quality Assurance samples in serum (n=6, range 100–2000 ng/mL) were sourced from Asqualab (Paris, France). Results were within the acceptable ranges provided by the scheme, with a mean difference of 2.6%.
720006049, July 2017