
In this application note, we use the ACQUITY UPC2 System coupled to ACQUITY SQD to analyze the identity and relationship of the unknown peaks observed during the method development standards and expired samples of metoclopramide.
UltraPerformance Convergence Chromatography (UPC2) exploits the benefits of sub-2-μm particle size stationary phases, with carbon dioxide as the primary mobile phase component. Convergence chromatography is a complementary analytical technique to liquid chromatography as it provides orthogonal selectivity, thereby increasing the opportunity to identify impurities present in a sample. Mass spectral information helps analysts confirm, identify, and characterize the quality of the pharmaceutical ingredients. Coupling UPC2 to mass spectrometry provides an important tool for pharmaceutical analysis compared to previously published reversed phase liquid chromatography (RPLC) impurity analysis approaches.1-3
Anomalies were observed during the method development screening process.4 In one instance, a standard solution of impurity F was hypothesized to be unstable after a few days. In this application, we use the ACQUITY UPC2 System coupled to ACQUITY SQD to analyze the identity and relationship of the unknown peaks observed during the method development standards and expired samples of metoclopramide. Impurity relationship to the API are hypothesized and confirmed with the use of the MS spectral data. Finally, the MS data from the impurity profile was interrogated to ensure the specificity of the methodology in the presence of these unknown peaks to aid future refinement of the final method.
|
UPC2 conditions |
|
|---|---|
|
System: |
ACQUITY UPC2 with PDA and SQD detection |
|
Column: |
ACQUITY UPC2 BEH 2-EP 3.0 x 100 mm, 1.7 μm |
|
Mobile phase A: |
CO2 |
|
Mobile phase B: |
1 g/L Ammonium formate in 50:50 methanol/acetonitrile spiked with 3% formic acid |
|
Wash solvents: |
70:30 methanol/ isopropanol |
|
Separation mode: |
Gradient; 5% to 30% B over 5.0 min; held at 30% for 1 min |
|
Flow rate: |
2.0 mL/min |
|
CCM back pressure: |
1500 psi |
|
Column temp.: |
50 °C |
|
Sample temp.: |
10 °C |
|
Injection volume: |
0.5 μL |
|
Run time: |
6.0 min |
|
Detection: |
PDA 3D Channel: PDA, 200 to 410 nm; 20Hz PDA 2D Channel: 275 nm at 4.8 nm Resolution (compensated 500 to 600 nm) SQD MS: 150 to 1200 Da; ES PosNeg |
|
Make-up flow: |
None; (make-up pump not configured in flow splitter) |
|
Data management: |
Empower 3 Software |
Investigations of impurity C and impurity F standards were prepared in methanol and explored at 0.1 mg/mL concentrations. The expired sample preparation was extracted using methanol, and prepared at a concentration of 2 mg/mL relative to the metoclopramide active ingredient.
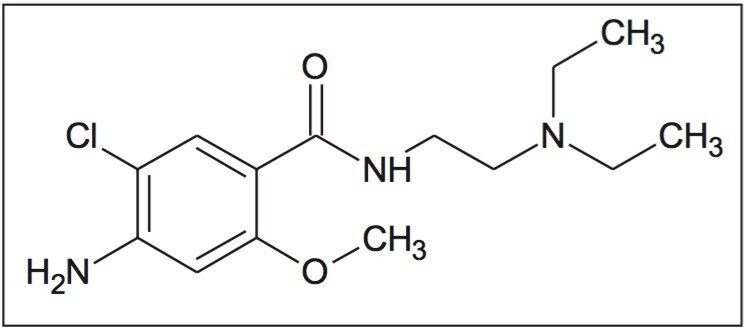
Prior to analyzing the degraded metoclopramide sample, the MS data was examined to address questions regarding observations with the chromatography of impurity reference standards 4-amino-5-chloro-2-methoxybenzoic acid; “impurity C” and 4-amino-5-chloro-N-2-(diethylaminoethyl0-2-hydroxybenzamide “impurity F.”
During the screening process, two peaks were separated during the injection of the impurity C standard using the ACQUITY UPC2 CSH Fluoro-Phenyl column. This phenomenon was not observed with the other stationary phases. MS spectral analysis of the two peaks showed similar spectra. The MS spectrum indicates possible dimerization of the analyte. Based on this information, it was determined that the two peaks were related to each other rather than the second peak being a contaminant. The rapid determination of this relationship provided direction for scoping future analysis by MS/MS and accurate mass. The data generated by those techniques will be more useful in determining the purity of the standard.
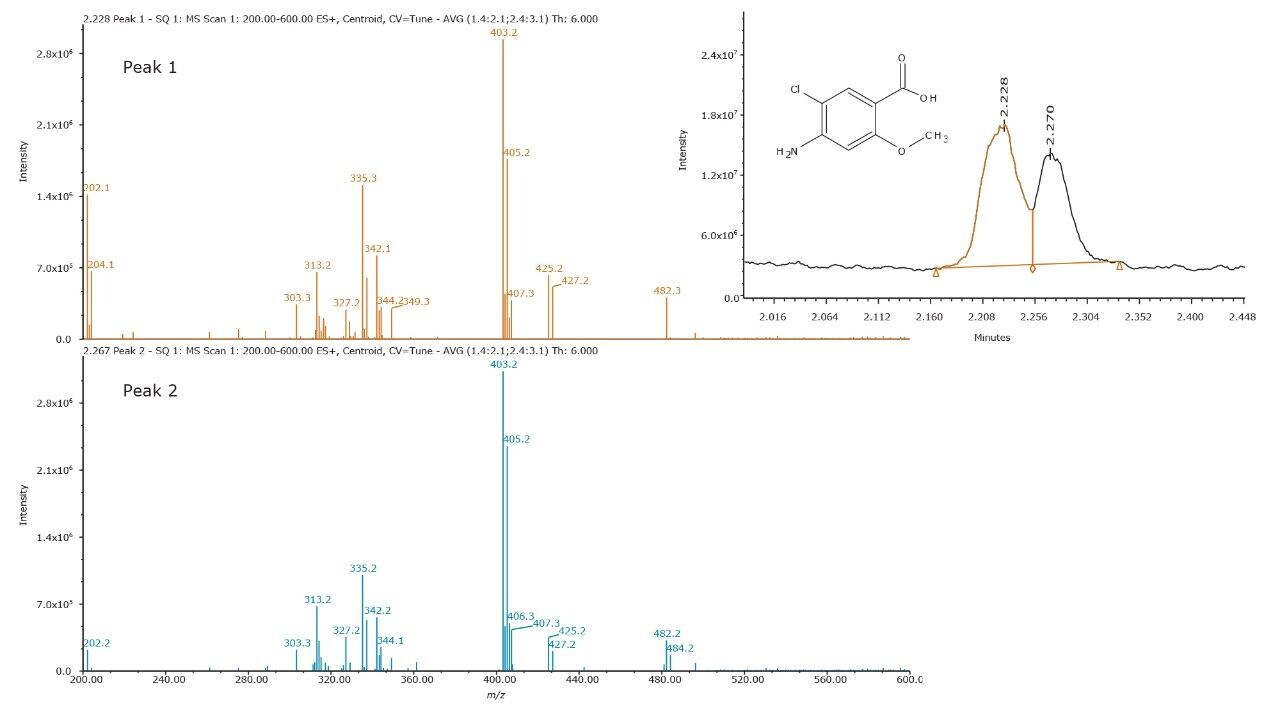
The peak shape of impurity F was observed to degrade over time, during the method development process. Unknown peaks would appear in the chromatogram within the course of a week. In addition, the color of the standard solution changed from a clear solution to a solution with a brownish tint. MS interrogation revealed the masses listed in Table 1. The masses were correlated to those found to be significant to the UV trace (not shown) between retention time 2.0 min and 3.5 min. XIC of m/z = 330 and 296 resulted in multiple peaks.
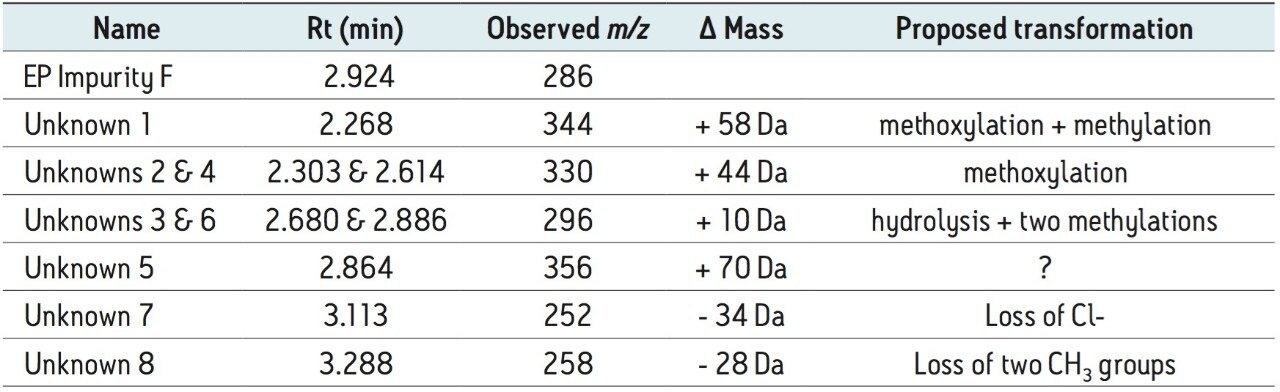
The working standard was prepared in methanol. Many of the impurity peaks were products of methylation or methoxylation. Based on this information, alternative diluents will be explored to inhibit the likelihood of these transformations. Presently, the working standard solution shelf life has been decreased to three days until a suitable diluent study can be performed. The unknown peak #8 in Table 1 with m/z = 258 has the same mass of methyl 4-(acetylamino)-5-chloro-2-methoxybenzoate, commonly referred to as “impurity B.” This unknown peak found in the impurity F working standard was determined not to be identical to EP impurity B due to differences in retention time; whereas, impurity B elutes at approximately 0.48 min. The origins of other impurities are still undetermined. They are hypothesized to be more products of solution instability or present in the reference material.
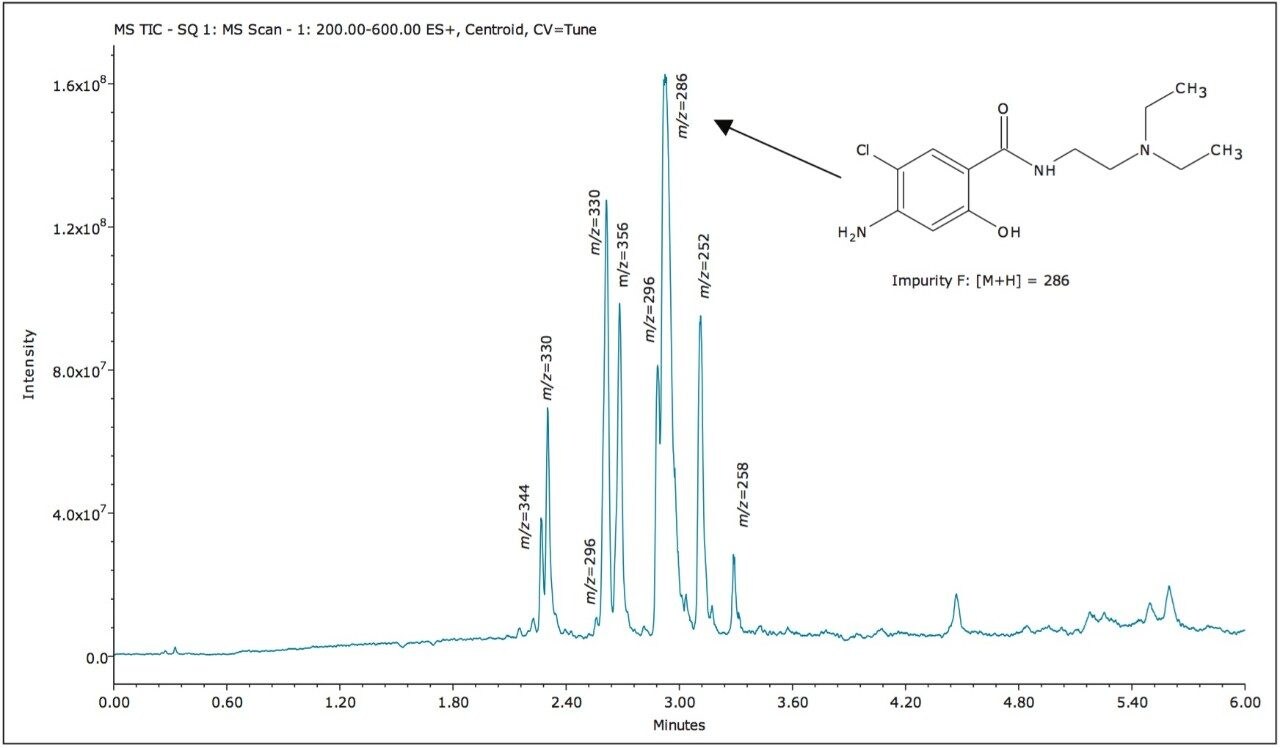
The ACQUITY UPC2 System coupled to the ACQUITY SQD Mass Spectrometer controlled by Empower 3 Software provided a simple solution to profile impurities in an expired metoclopramide sample. MS spectral extraction of peaks in the UV chromatographic trace were simply performed by using right mouse click in the review window to rapidly confirm known impurities and identify 12 unknown impurities, as shown in Table 2. The sensitivity of UPC2 provided s/n ≥ 10 for impurities detected with area% ≥ 0.05% in the UV chromatographic trace. The expired metoclopramide sample was interrogated to determine if the masses in Table 1 were present. The MS data confirmed the presence of 4 out of the 8 impurities related to EP F; m/z =296, 344, 252, and 258. In addition to the known impurities, a total of 7 unknown impurities were detected. The masses, retention time, and UV signal-to-noise were recorded in Table 2.
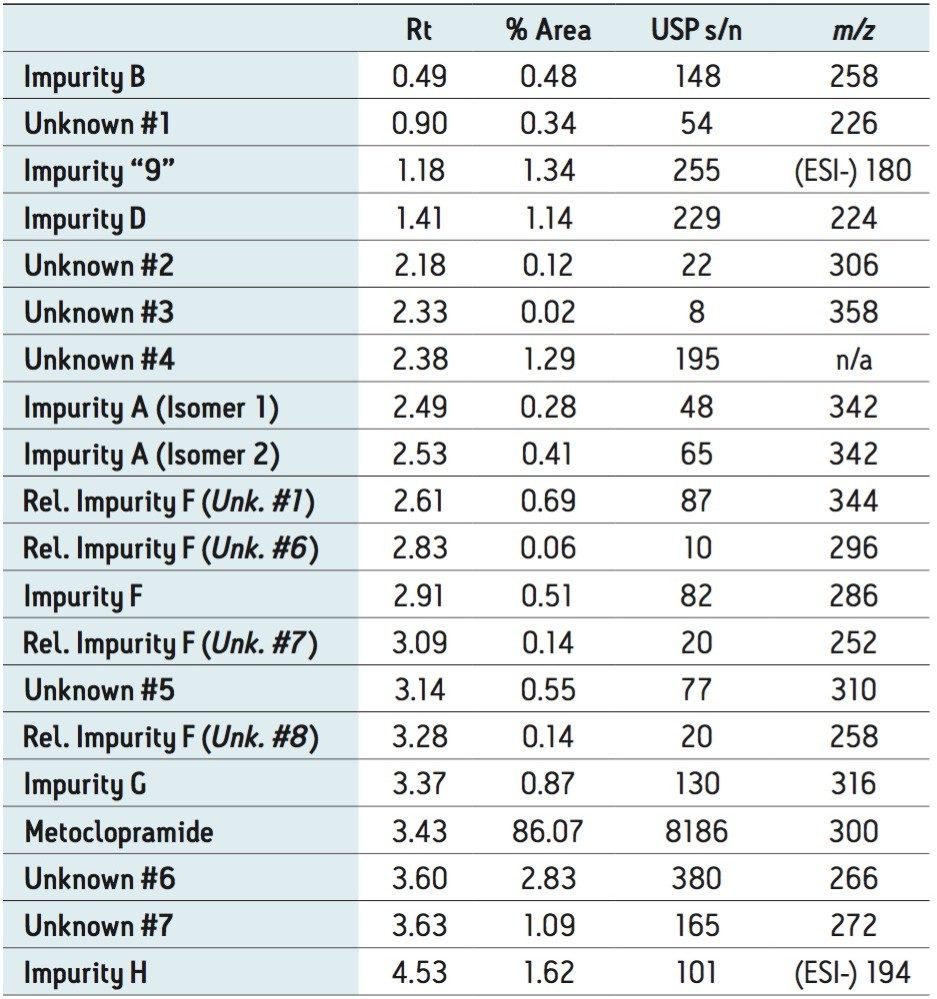
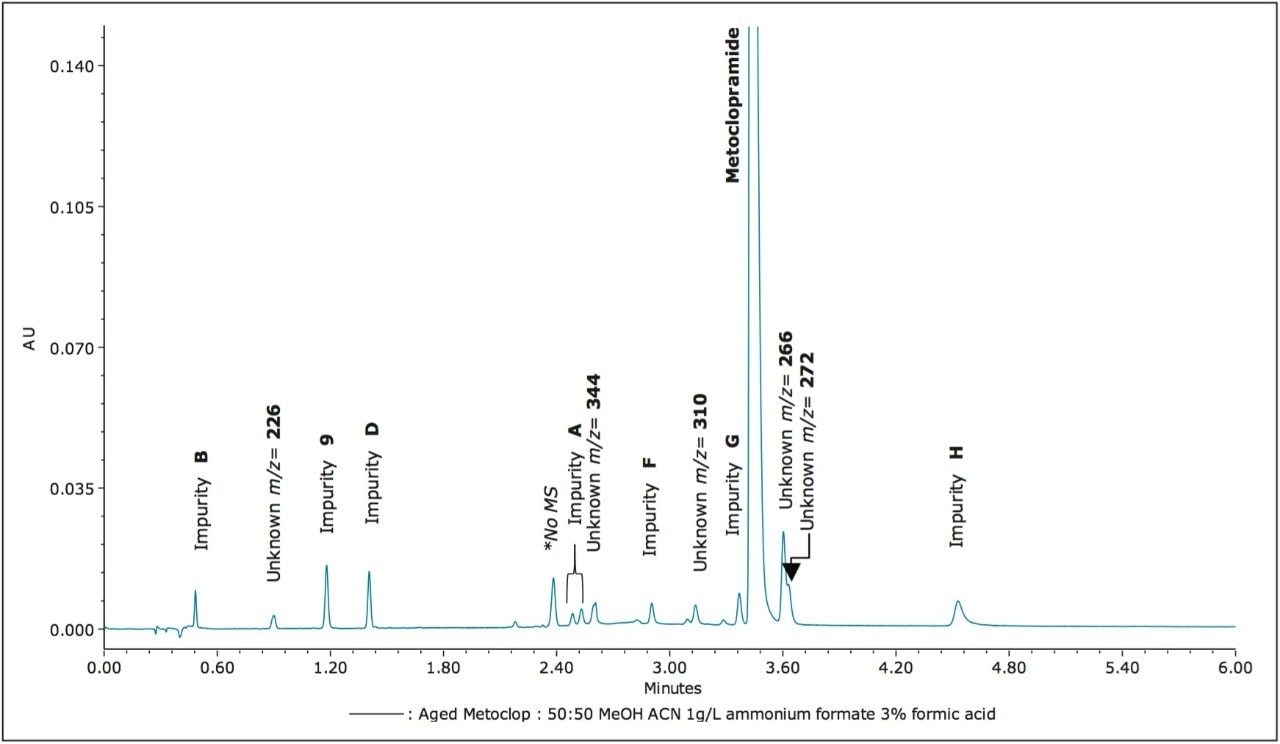
The ACQUITY UPC2 System coupled to MS provided a comprehensive approach to impurity profiling. This configuration enables a scientist to quickly answer questions regarding reference standard purity, as shown with reference standard impurity C. UPC2-MS guided the decisions to investigate diluent choices for the impurity F working standard and adjusting the shelf life of the working standard solution. In addition, investigating instability of impurity F provided insight into other potential impurities that may be present in the drug sample impurity profile. Furthermore, seven unknown impurities were detected in the expired metoclopramide sample. Interrogation of the UV and MS data was simply performed using Empower 3 Software. Overall, utilizing UPC2-MS increased the knowledge base of pharmaceutical product quality, and improved the methodology procedures involved with achieving the analytical goals.
720004575, February 2013