This is an Application Brief and does not contain a detailed Experimental section.
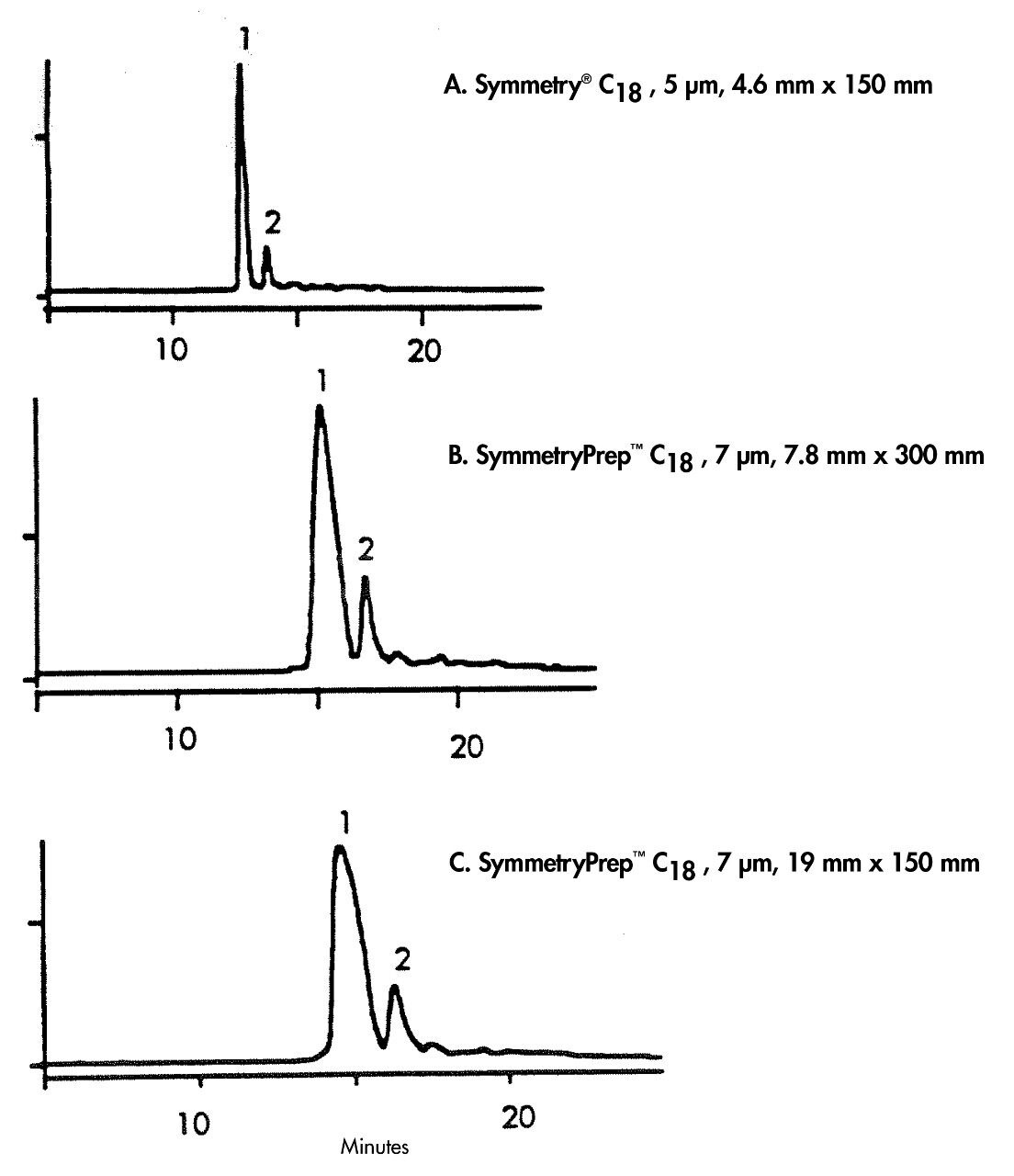
This application brief demonstrates analysis of insulin- impurity isolation, transfer from analytical to prep.
The compounds used in this study are –
|
Columns: |
A. Symmetry C18, 4.6 x 150 mm, 5 μm B. Symmetry Prep C18, 7.8 x 300 mm, 7 μm C. Symmetry Prep C18, 19 x 150 mm, 7 μm |
|
Part numbers: |
A. WAT045905 B. WAT066235 C. WAT066240 |
|
Mobile phase: |
A. 0.1% TFA in Water B. 0.1% TFA/acetonitrile 26% B to 33% B in 14 minutes adjusted for gradient delay on prep system |
|
Flow rates: |
A. 1.0 mL/min b. 5.76 mL/min c. 17mL/min |
|
Detection: |
UV @ 280 nm |
|
Sample load: |
Bovine pancreas insulin, 10 mg/mL A. 0.07 mg B. 3 mg C. 8.5mg |
|
HPLC systems: |
A. analytical system B. and C. prep system with 0.04 in. I.D. tubing |
WA31763.92, June 2003