High-Performance DESI-MSI using a DESI XS Xevo™ TQ Absolute XR system for Sensitive and Specific Targeted Imaging of Small Molecules in Tissue Sections
Emmanuelle Claude
Waters Corporation, Wilmslow, United Kingdom
Published on September 23, 2025
Abstract
Desorption Electrospray Ionization Mass Spectrometry Imaging (DESI-MSI) has emerged as a powerful, minimal-preparation method to ionize and image small molecules—especially phospholipids—directly from tissue sections. High spatial resolution MSI comes at the cost of relatively long acquisition times, so system stability and robustness are paramount.
The system solution described in this study combines an ACQUITY™ µBSM binary pump for ultra-steady solvent flow, the DESI High-Performance Sprayer (HPS) for spray stability, and a DESI XS source on a Xevo TQ Absolute XR for highly sensitive, selective MRM-based imaging of lipids and small endogenous metabolites in rodent brain tissue sections.
Benefits
- The sensitive and specific mapping of endogenous small metabolites and lipids in rodent brain using targeted MS imaging workflows
- Robustness of targeted DESI MSI using an ACQUITY µBSM binary pump DESI solvent delivery combined with the DESI XS source on a Xevo TQ Absolute XR mass spectrometer
- Superior specificity and sensitivity of targeted MS imaging DESI workflows for detecting and visualizing low abundance molecules directly from surfaces
- Illustrating the application of targeted MS imaging workflows with MRM acquisition to visualize isobaric and isomeric species through the use of diagnostic product ions
Introduction
Over the past decade, DESI has become a growing technique for directly ionizing and imaging small molecules from tissue sections with virtually no sample preparation. Phospholipids draw intense interest across biology and medicine because they underpin membrane structure and fluidity, and govern key processes such as signalling and molecular transport. Also, shifts in phospholipid profiles can flag disease states, metabolic dysregulation or inflammatory responses. In particular, studying brain, where they are especially abundant—they inform studies of development, aging and neurodegeneration (e.g. Alzheimer’s, Parkinson’s diseases).
Furthermore, by mapping endogenous small molecules in situ, DESI-MSI exposes regional metabolic and signalling changes that conventional bulk assays simply can’t resolve. Generating higher spatially resolved DESI imaging in the low tens of microns is becoming a necessity to better understand tissue margins, microenvironmental heterogeneity or metabolic region-specific shift. This can induce longer acquisition times and therefore system stability and robustness are key factors.
In our workflow, an ACQUITY µBSM binary pump is harnessed to deliver steady DESI solvent flow rate combined with the DESI HPS for a stable spray and a DESI XS source on a Xevo TQ Absolute XR Mass Spectrometer for the sensitivity and specificity of the targeted MRM-MS imaging mode.
Experimental
Sample Preparation
Consecutives rodent brain tissue sections were generated from snap-frozen tissue that were stored at -80 °C, using a cryostat (Leica) at 18 µm thickness and thaw-mounted onto standard microscope slides (1 x 3 inches). Tissue sections were kept at -80 °C until analysis.
Mass Spectrometry
Experiments were performed using a DESI XS source mounted on a Xevo TQ Absolute XR Triple Quadrupole Mass Spectrometer in MRM mode of acquisition. The DESI spray conditions were set at 2 µL/min, 98:2 MeOH/H2O using an ACQUITY µBSM binary pump and a N2 nebulizing gas pressure of 15 psi.
A DESI HPS was utilized for improved sensitivity, DESI spray focus, robustness and ease-of-use. Furthermore, a heated transfer line (HTL) was mounted directly onto the ion block of the TQ Absolute XR. It was set at room temperatures for the small molecules experiments and heated to 450 °C for the negative mode isomeric phospholipid experiment. This enhanced the desorption and transfer of the charged droplets into the mass spectrometer resulting in increased sensitivity.
MS Conditions
|
MS systems: |
Xevo TQ Absolute XR Mass Spectrometer |
|
|
Source type: |
DESI XS |
|
|
Polarity: |
Positive and Negative |
|
|
Source temperature (°C): |
150 |
|
|
MS1 resolution: |
Unit (0.7 Da) |
|
|
MS2 resolution: |
Unit (0,7 Da) |
|
|
Dwell time: |
6 ms |
|
|
Experiment type: |
MRM |
DESI Setup
|
Capillary voltage (kV): |
0.70 (negative) and 0.75 (positive) |
|
|
Nitrogen flow (psi): |
15 |
|
|
Solvent delivery: |
ACQUITY µBSM binary pump |
|
|
Solvent: |
98% Methanol, 2% Water |
|
|
Solvent flow rate: |
2 µL/min |
|
|
Pixel size: |
25 and 35 µm |
Data Mangement
|
MS software: |
MassLynx™ v4.2 Software (SCN 1050) (For Xevo TQ Absolute XR) |
|
Informatics: |
High Definition™ Imaging (HDI™) 1.8 |
Results and Discussion
A) Phospholipids
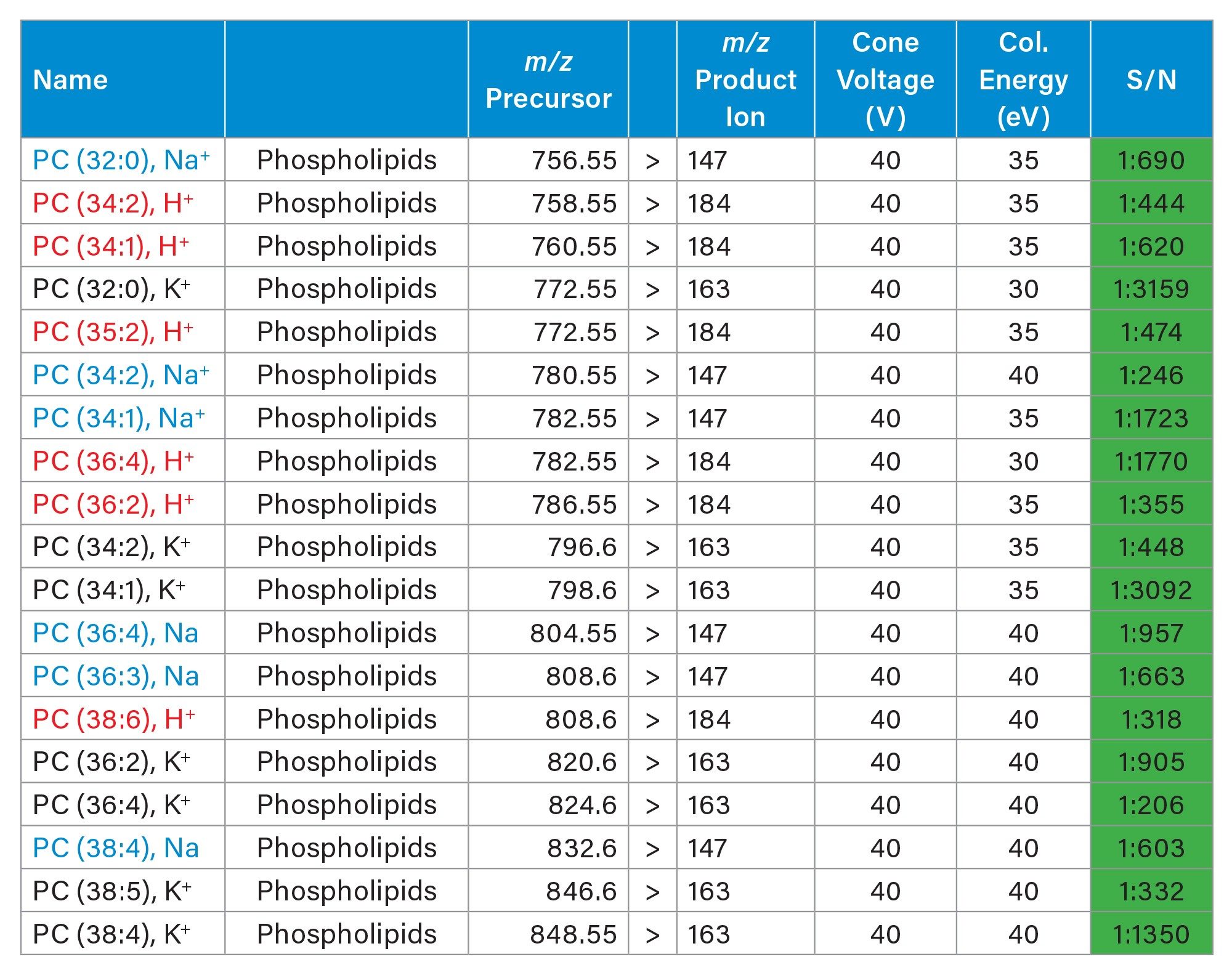
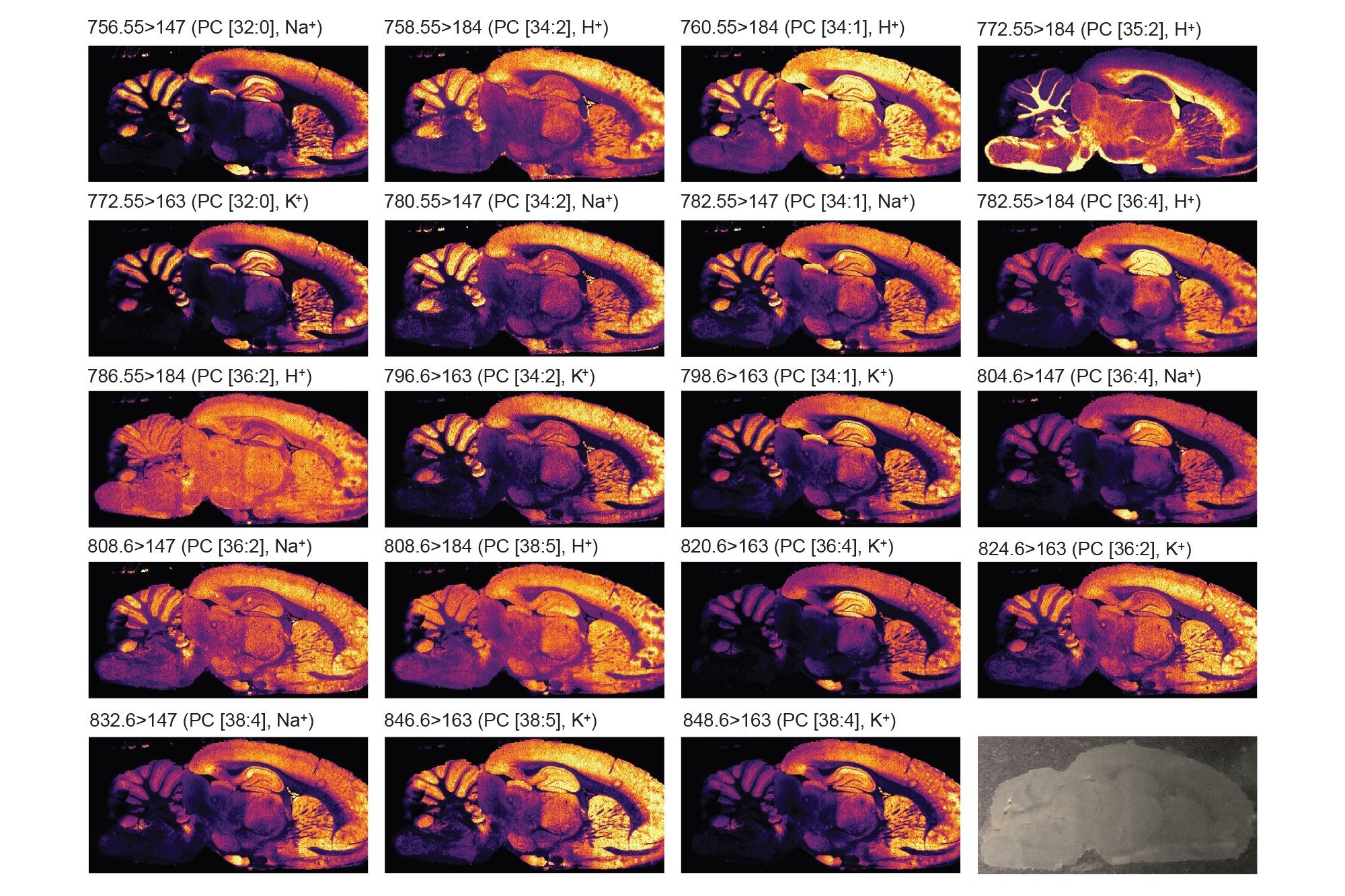
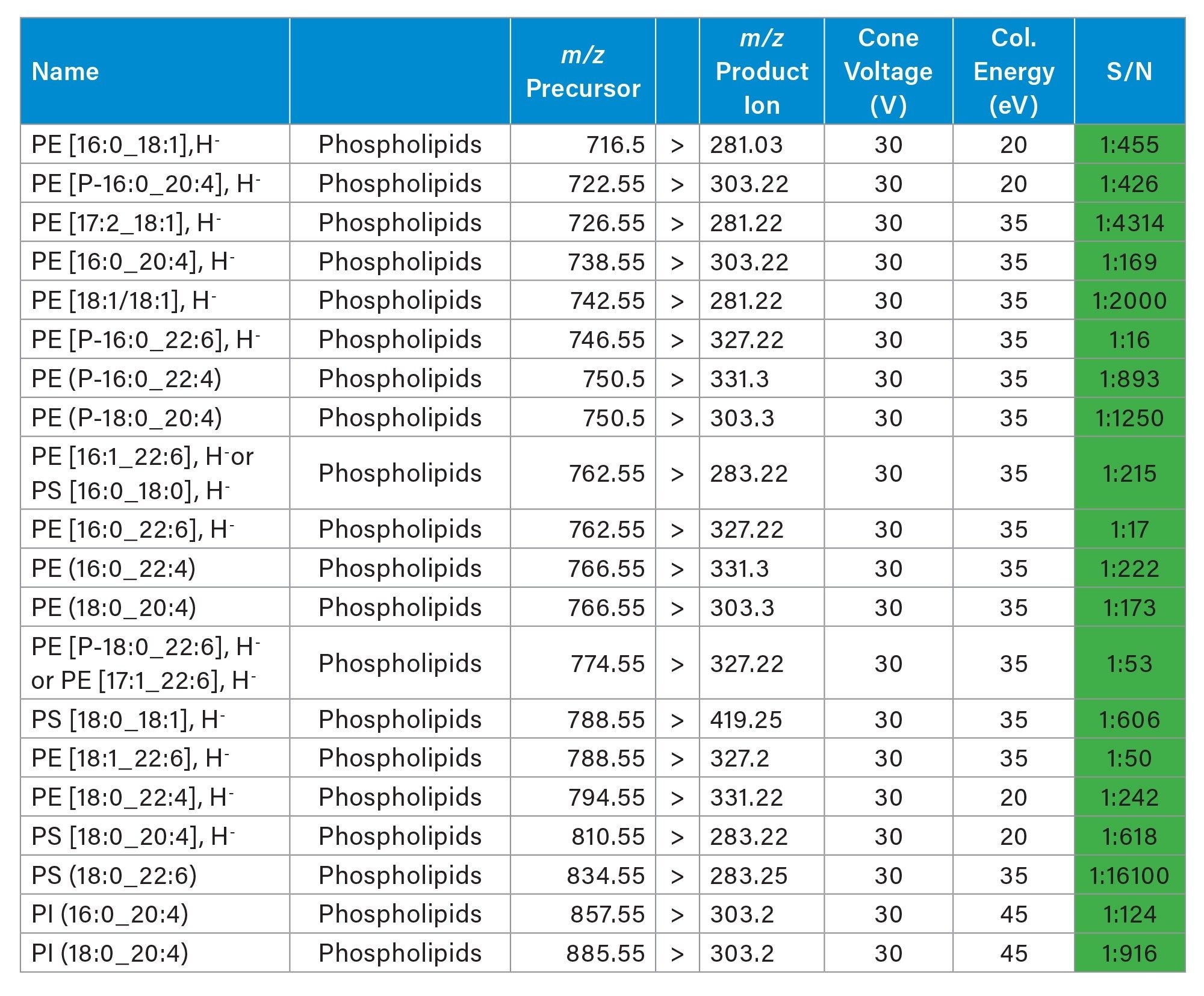
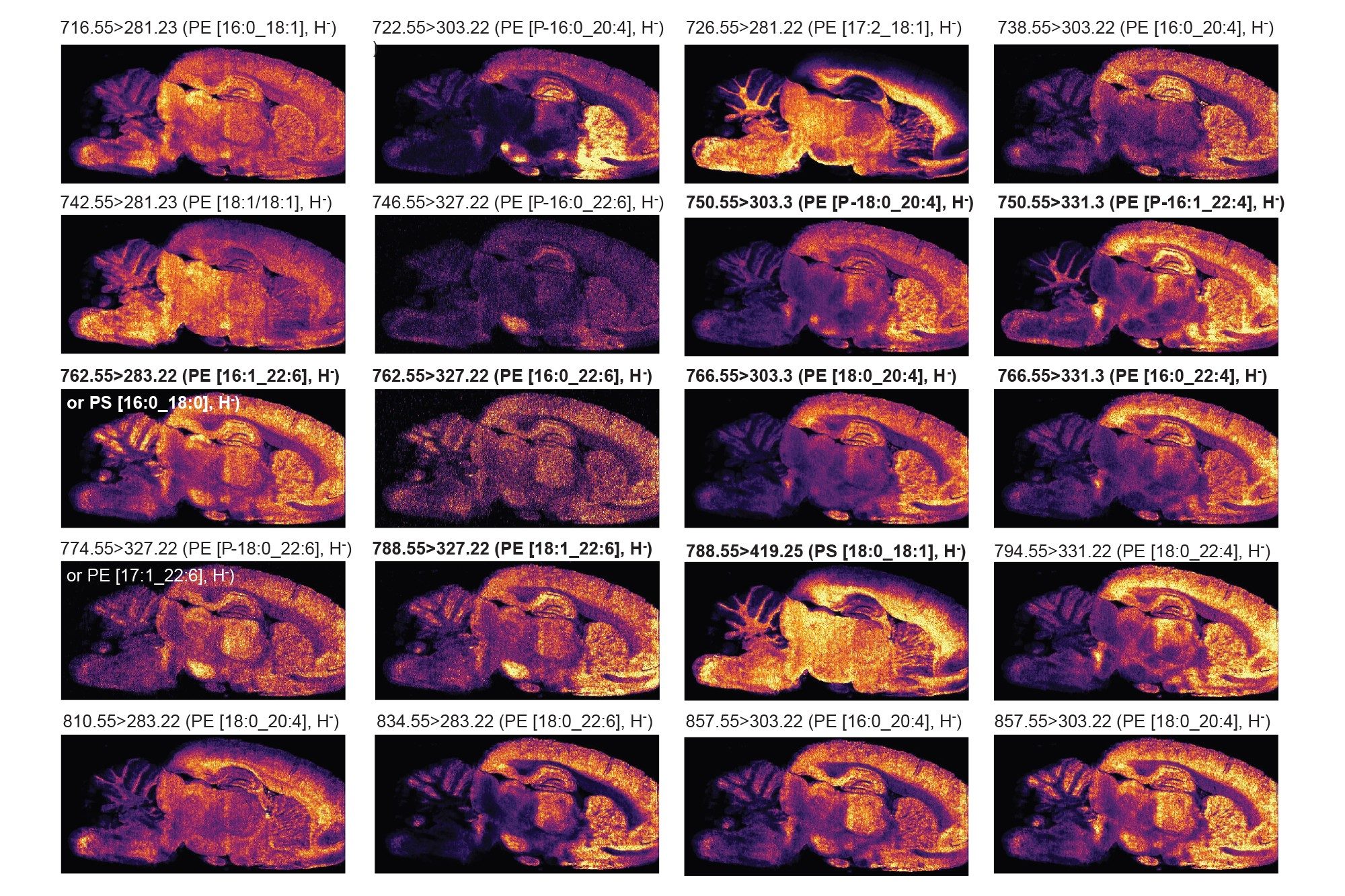
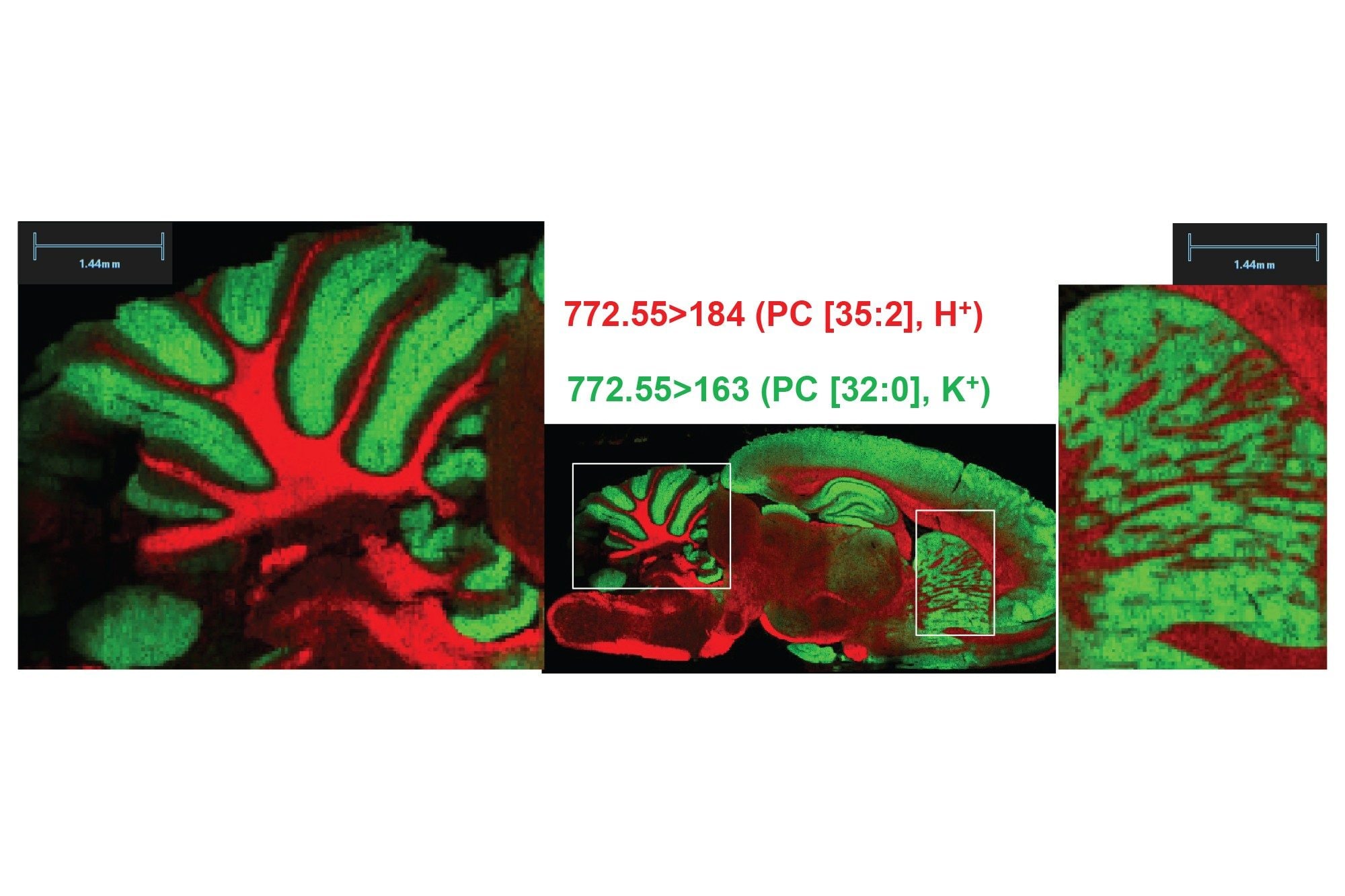
Two analyses of the same rodent brain tissue section have been performed visualizing phospholipids in positive and negative ionisation mode by combining the ACQUITY µBSM binary pump and DESI XS mounted onto the Xevo TQ Absolute XR. The acquisition speed was 5 scans/seconds, and the total time of acquisition were approximately 11-12 hours.
As seen in figures 1 and 2, the non-normalised ion images show the great signal stability and robustness of the whole system from the steady solvent delivery using the ACQUITY µBSM, the DESI HP sprayer and Xevo TQ Absolute XR Mass Spectrometer. Furthermore, the signal to noise (S/N) for the targeted lipids are reported in table 1 and 2 alongside the MRM transition information, where some of them were isobaric and/or isomeric such as lipids m/z 772.55 in positive ionisation mode and lipids m/z 788.55 in negative ionisation mode.
B) Small molecule metabolites
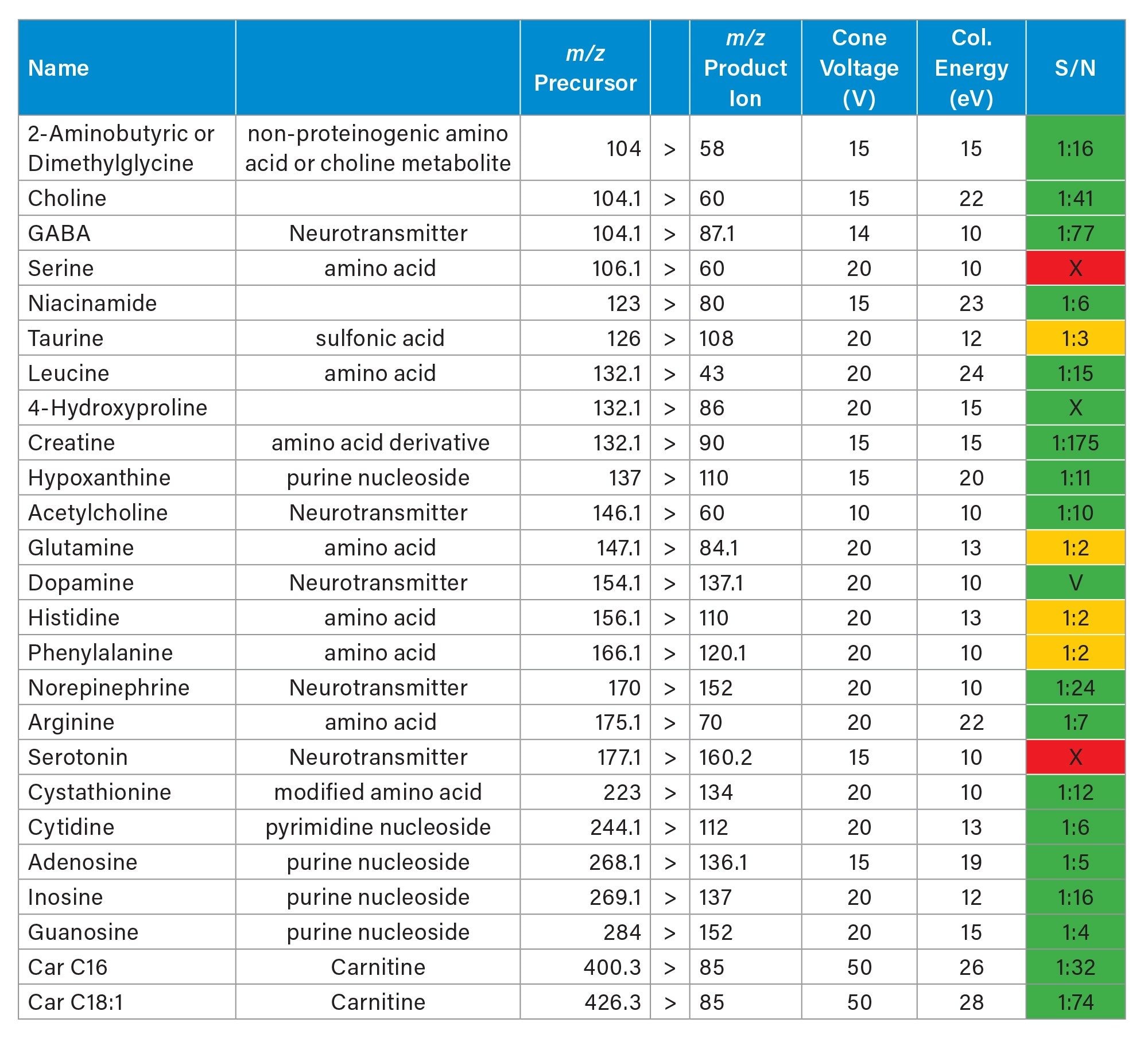
Further experiments were conducted analysing consecutive rodent brain tissue sections, targeting endogenous small molecules in negative and positive ionisation mode.
Firstly, in negative mode, three experiments were performed targeting some amino acids, TCA cycle metabolites, arachidonic acid metabolites (which are also called oxylipins or lipid mediators) and fatty acids. Each experiment lasted 11-13 hours. In table 3, 4 and 5 the MRM transitions and S/N are reported. In table 4, some of the HETEs S/N using the Xevo TQ Absolute XR are similar to those ones reported when coronal rodent tissue sections were analysed using a previous iteration of the DESI MSI System (a Xevo TQ Absolute Mass Spectrometer)1.
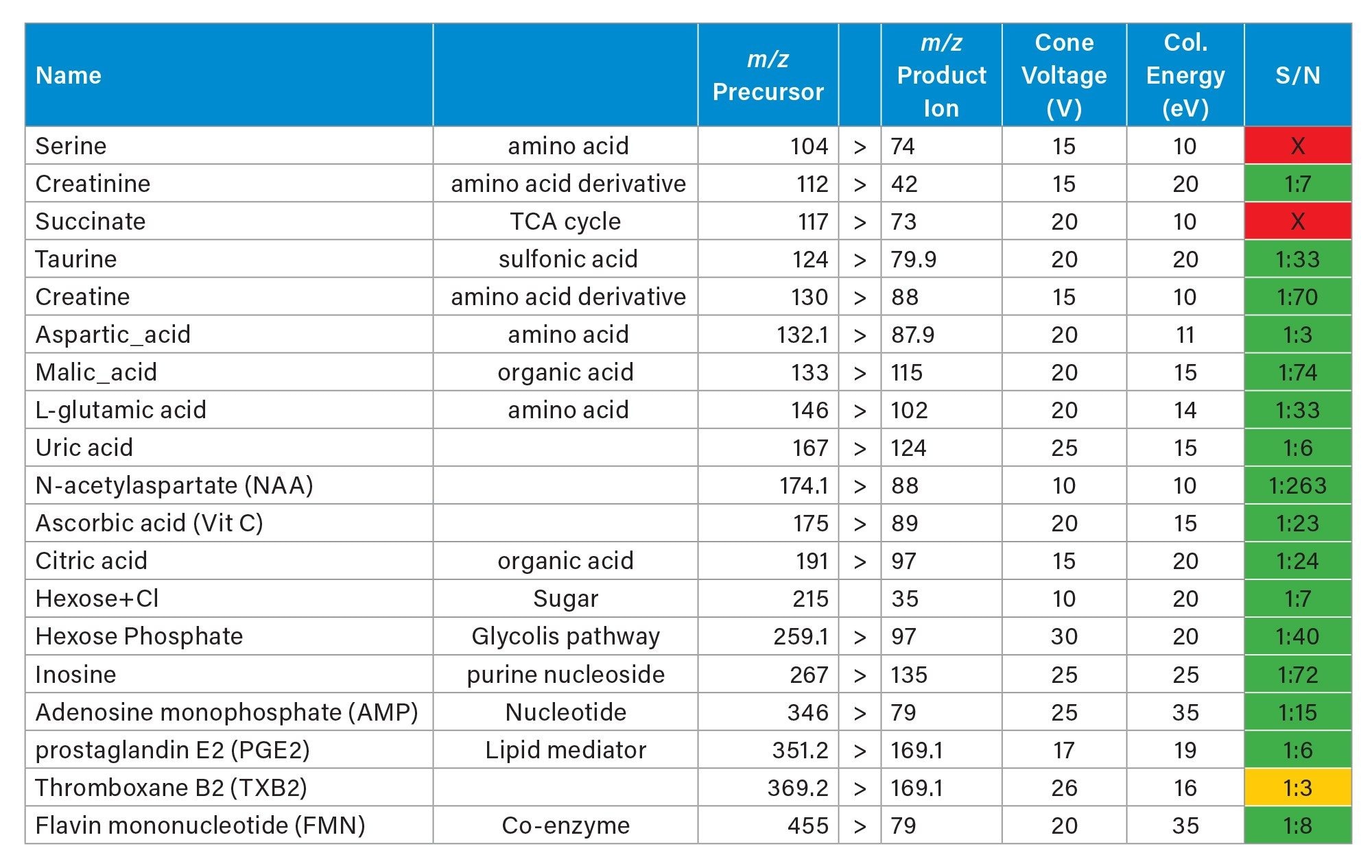
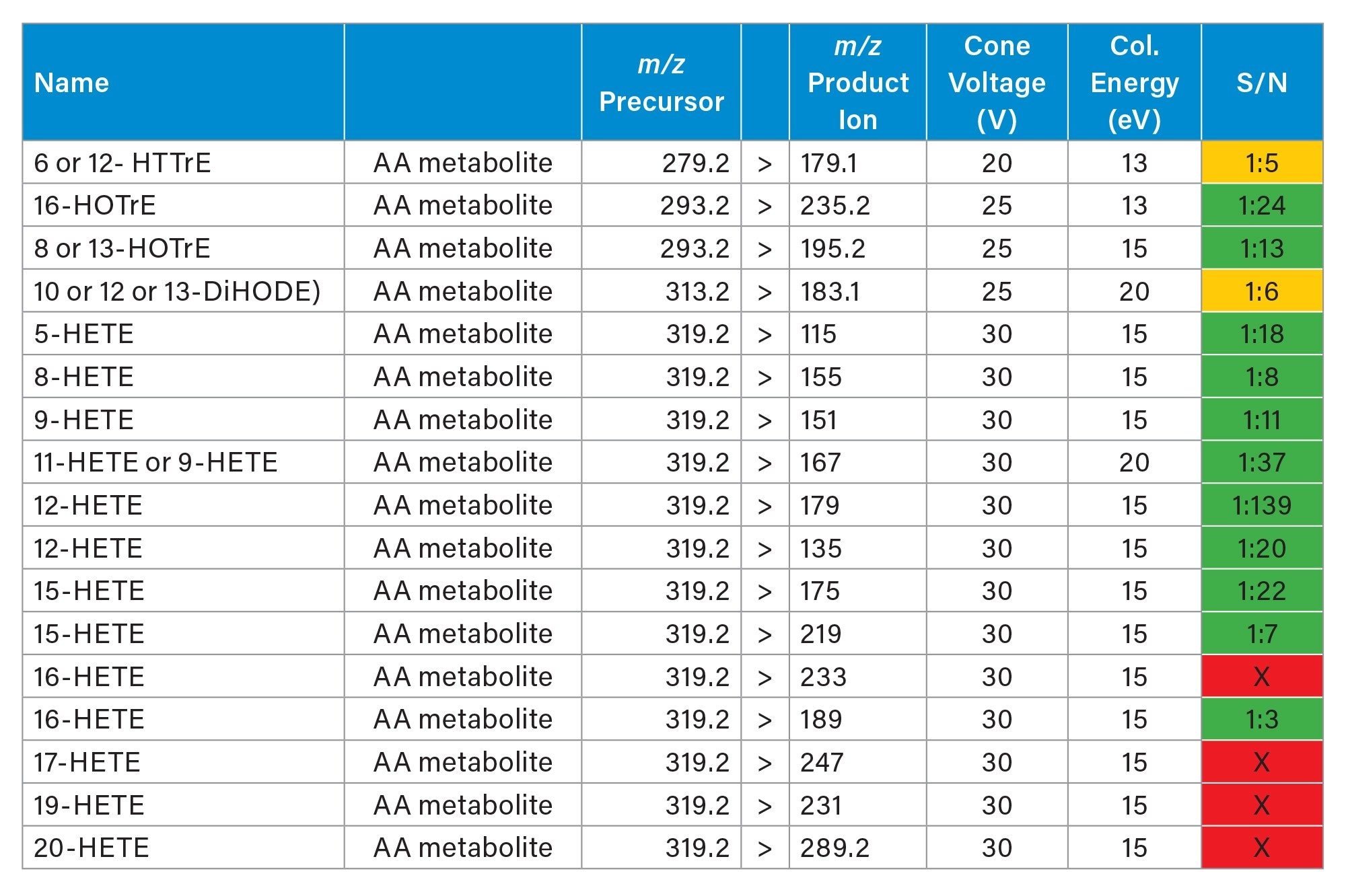
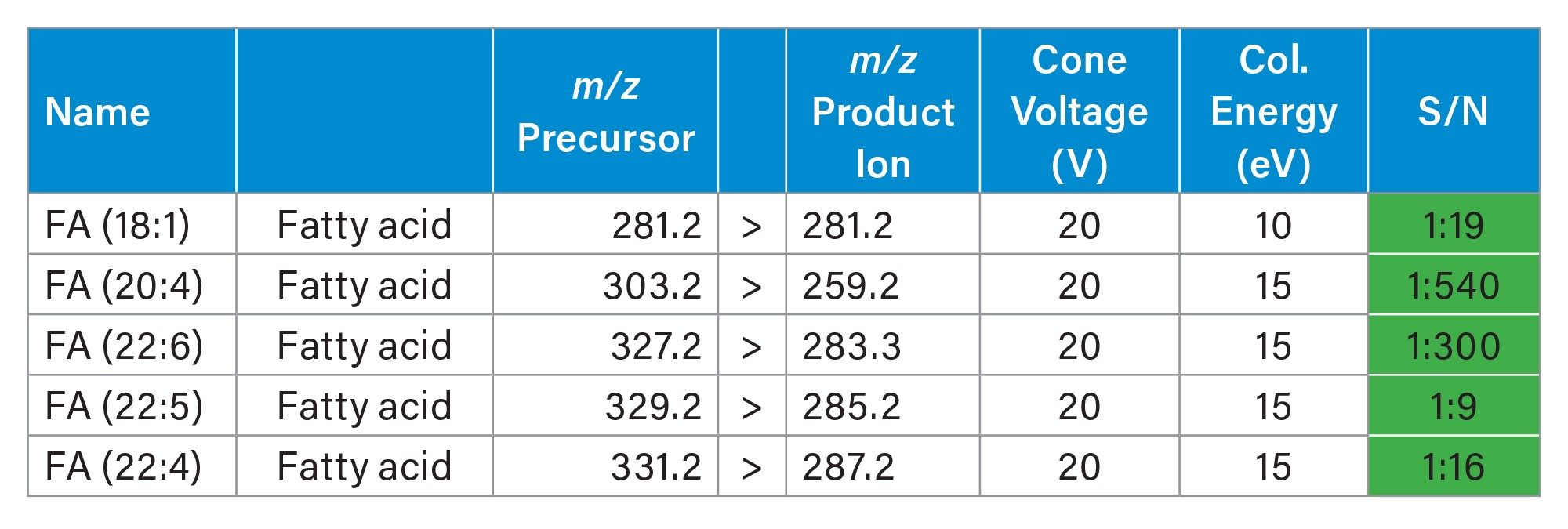
Figure 4 displayed some examples of the distributions of a panel of small endogenous molecules in rodent brain tissue sections.
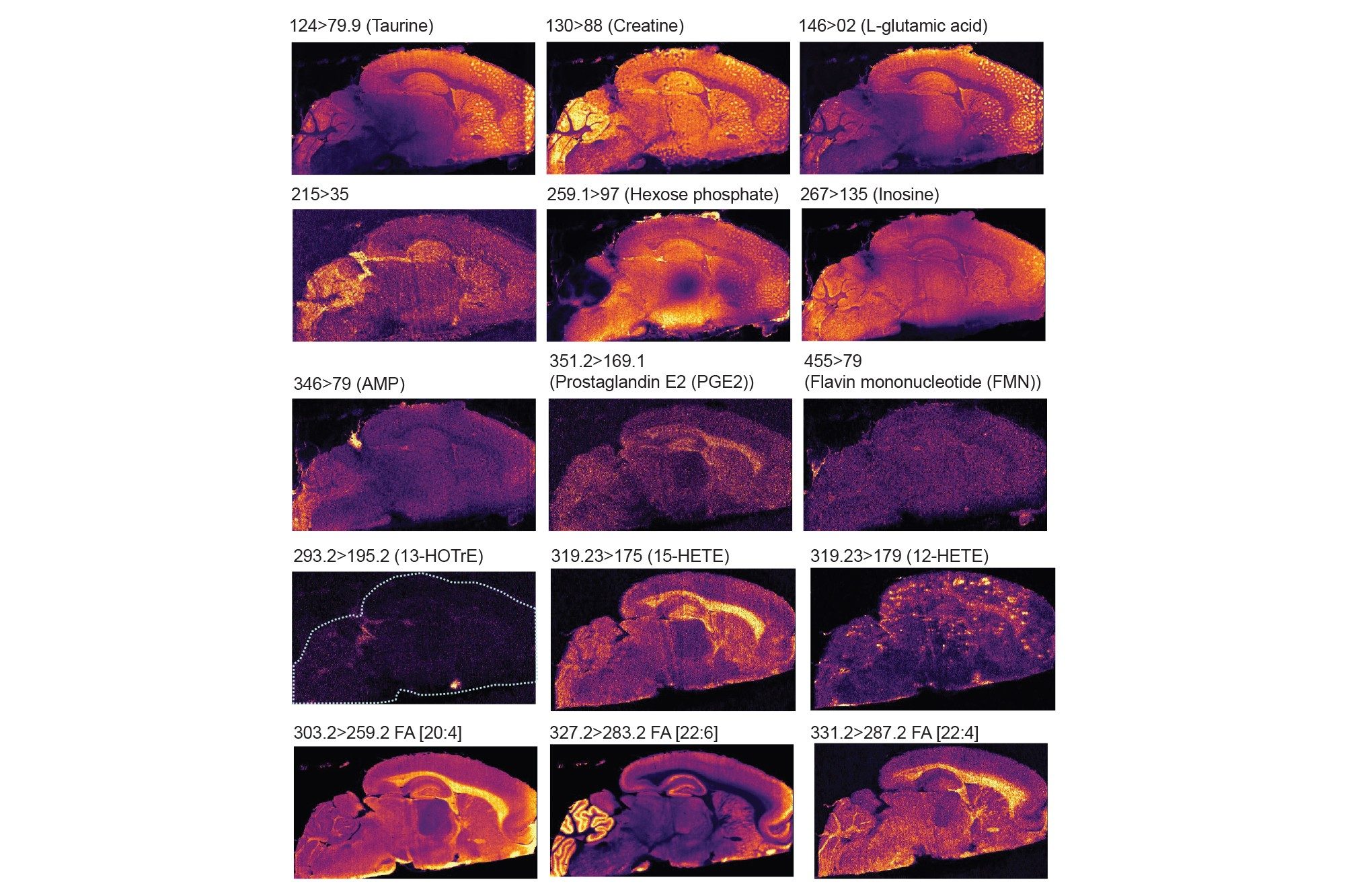
A further two experiments were carried out in positive ionisation focusing more on small metabolites that ionise preferentially in positive ionisation mode, such as certain amino acids, neurotransmitters, carnitine. A selection of their distributions are displayed in figure 5.
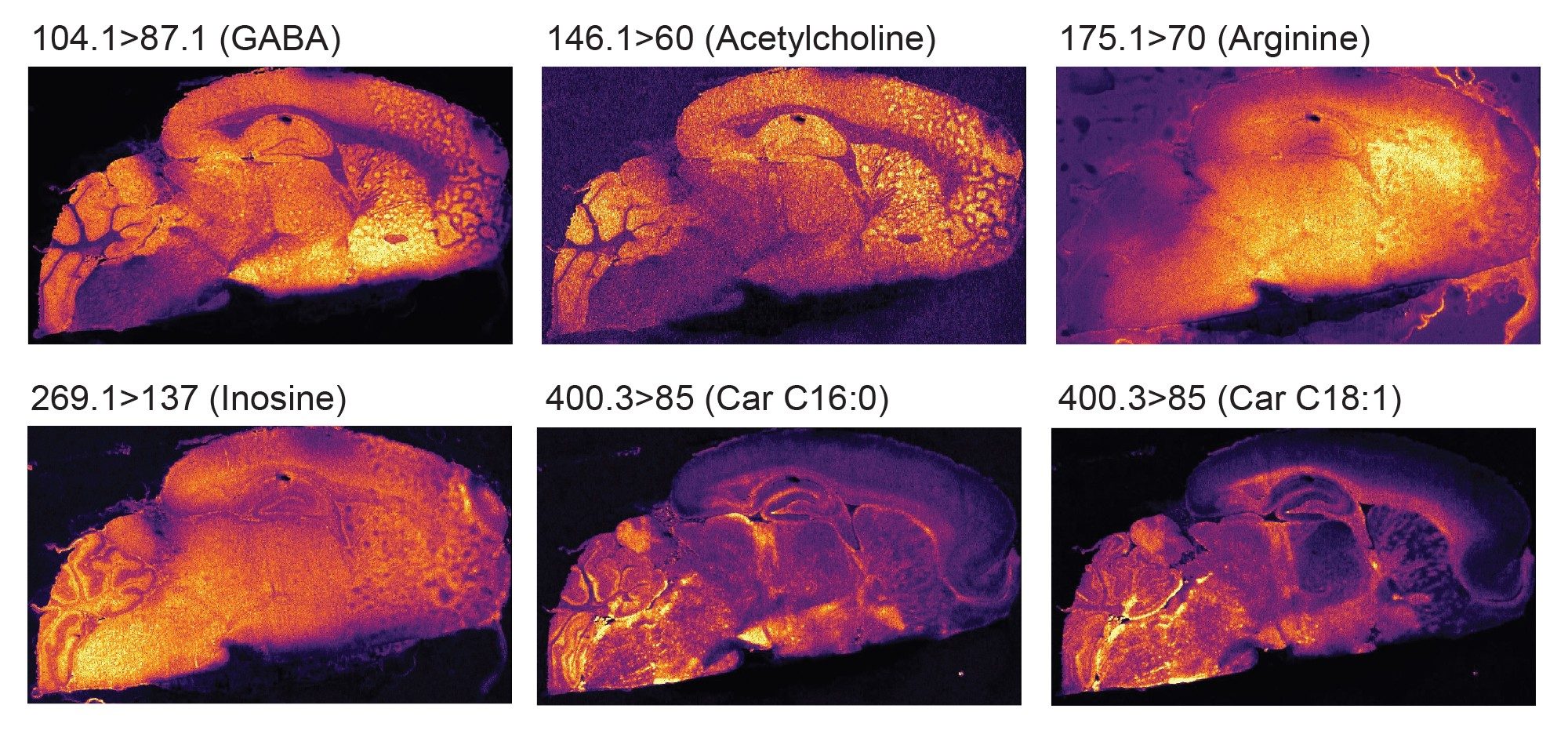
Conclusion
The targeted MS imaging workflow combining a ACQUITY µBSM binary pump, DESI HPS, and a DESI XS source on a Xevo TQ Absolute XR Mass Spectrometer was demonstrated to deliver many hours of signal stability for the consistent generation of high-quality ion images over long periods of time.
Low abundant small molecules were detected directly from brain tissue section thanks to the sensitivity of the Xevo TQ Absolute XR Mass Spectrometer. Moreover, the specificity of the system allowed the differentiation of isobaric and isomeric molecules such as phospholipids, oxylipins, and small metabolites.
References
- Using Targeted MRM MS Imaging With DESI to Visually Localize Isobaric And/or Low Abundance Lipids, Waters application note. 720008692.
720009047, September 2025