Determination of Drugs of Abuse in Whole Blood Using the Ostro™ Pass-through Sample Preparation Plate
For forensic toxicology use only.
This is an Application Brief and does not contain a detailed Experimental section.
Abstract
The use of whole blood, as a biological matrix for forensic testing, has been popular for many years. As whole blood is considered a complex matrix, any analytical workflow should include a robust sample preparation method. Here we describe a simple pass-through sample preparation method. The method has been applied to a large panel of drug substances which covers differing chemical classes and will support a range of forensic toxicology testing scenarios.
Benefits
- Single generic sample preparation protocol using an Ostro Pass-Through Sample Preparation Plate
- Precipitation, filtration, and sample clean-up in one device
- Multiple drug classes separated on either a BEH™ C18 ACQUITY™ UPLC or HSS C18 ACQUITY Column using an ACQUITY UPLC I-Class
- Excellent sensitivity of the Xevo™ TQ-S micro mass spectrometer allows for the analytes to be detected at toxicologically relevant concentrations
Introduction
Forensic toxicology deals with the investigation of drugs or toxic substances within a biological specimen. To this end, laboratories require reliable methods that can detect a wide variety of these toxicants in postmortem and antemortem specimens.
The use of whole blood as a biological matrix for forensic testing has been popular for many years. For example, it is the matrix of choice for investigations into probable cause of death, where analytical protocols commonly involve the application of general unknown screening procedures (also known as ‘systematic toxicological analysis’) that are designed to screen for potentially hundreds of xenobiotics. These may be banned or illicit drugs, emerging psychoactive substances, or prescribed and over-the-counter medications.
Blood is also the most frequently used specimen for confirmatory analysis where recent drug ingestion is suspected, for example, in investigations into driving under the influence (DUID), as the quantitative measurement of drugs in blood is considered to provide the most accurate picture of the current effects of drugs on the body as some blood concentrations are known to correlate well with impairment. As driving under the influence is a significant concern globally, most countries around the world now have legislation in place to improve road safety and to decrease motor vehicle accidents. Typically, this involves monitoring a defined panel of specific drug substances. For example, in England and Wales, analytical methods currently target a panel of 17 specific drug substances to support the requirements of Section 5A of the Road Traffic Act 1988,1 while in the United States the National Safety Council’s Drugs and Impairment Division recommends a panel of compounds that it considers crucial for monitoring in drivers (Tier 1 compounds) as these substances are frequently found in arrests associated with impaired driving.2 However, as there is an awareness that there are many other drug substances, or combinations of drugs, that can pose the risk of impairment, a much wider panel of substances is also of interest for more comprehensive testing as countries continue to adapt their own testing protocols and recommendations for monitoring drivers, depending on the drugs used/prescribed in their specific region and their prevalence of use.
Forensically relevant substances can be quite diverse, covering different chemical classes and ranging from polar bases, such as opiates and amphetamines to non-polar acids, such as non-steroidal anti-inflammatory drugs, therefore when monitoring these substances the laboratory will need to employ a generic sample preparation method that is capable of extracting all of these different chemical classes from the whole blood, while still providing some clean-up of the sample prior to the use of sensitive analytical procedures such as UPLC™-MS/MS.
Previously we have described a simple clean-up protocol for whole blood based on Waters Ostro Pass-Through Sample Preparation Plate.3 Ostro combines removal of proteins and phospholipids and filtration in one device. The method was applied in combination with UPLC-MS/MS methods for the analysis of the specific panel of 17 drugs to support the specific requirements of the Section 5A of the England and Wales Road Traffic Act 1988. In this study we apply the same protocol to much wider panel of drug substances that will support a broader range of forensic testing scenarios.
Experimental
Control human whole blood (K2 EDTA, pooled) was supplied by Bio-IVT (Burgess Hill, West Sussex, UK).
Reference material for toxicologically relevant substances were obtained from Merck (Poole, Dorset, UK), LGC (Teddington, London, UK). These were supplied as individual 1mg/mL solutions in either methanol or acetonitrile. Analytes were combined to produce several mixed-drug spiking solutions in methanol.
Control whole blood was spiked with a range of drugs that covered the following groups at the following concentrations: the analytes which ionize in electrospray positive mode (ESI+) were spiked at 100 ng/mL, those which ionize in electrospray negative (ESI-) mode were spiked at 200 ng/mL and the cannabinoids were spiked at 10 ng/mL. Aliquots of the blood was prepared as previously described.3 In brief, an aliquot (100 µL) of control or spiked blood was added to 100 µL zinc sulphate/ammonium acetate solution in the well of an Ostro Sample Preparation 96-well Plate (p/n: 186005518) and briefly mixed. Elution solvent (600 µL of 0.5% formic acid in acetonitrile) was added to the wells and the plate vortex-mixed for three minutes. The plate was placed onto a vacuum manifold and the elution solvent was drawn into a Waters 2 mL Square-well Collection Plate (p/n: 186002482). Three separate aliquots (3 x 150 µL) of the eluant were transferred to a Round-well Collection Plate (p/n: 186002481) and taken to dryness using an Ultravap Mistral microplate evaporator (Porvair). Dried wells were reconstituted by addition of 50 µL of the appropriate solvent for the specific UPLC-MS/MS method used to analyze the samples, as detailed below.
The drugs that ionize in ESI+ mode were analyzed by a UPLC-MS/MS method using an ACQUITY HSS C18 Column (2.1 x 150 mm, 1.8 µm, p/n: 186003534) with mobile phases containing 0.1% formic acid in water and acetonitrile. The reconstitution solvent for this assay was 5% acetonitrile in 0.1% formic acid.
The drugs that ionize in ESI- mode were analyzed on the same ACQUITY HSS C18 Column but with mobile phases containing 0.001% formic acid in water and 0.001% formic acid in acetonitrile. The reconstitution solvent for this assay was 10% acetonitrile in 0.001% formic acid.
A separate method, for the measurement of cannabinoids was based on the ACQUITY BEH C18 Column (2.1 x 100 mm, 1.7 µm; p/n: 186002352) with mobile phases containing 0.05% formic acid in water (mobile phase A) and 0.05% formic acid in acetonitrile (mobile phase B). The reconstitution solvent for this assay was 0.05% formic acid in acetonitrile.
For each UPLC-MS/MS method 2 MRM transitions (where possible) were monitored for each analyte.
Results and Discussion
In total 155 analytes were investigated using the developed sample preparation procedure, these included 110 substances that were analyzed using a broad MRM screening method in ESI+, 40 analyzed using a broad screen MRM in ESI- and 5 cannabinoids detected using a dedicated MRM with switching between ESI+/ESI-.
All substances investigated in this study were detected at the investigated concentrations and are listed in Table 1.
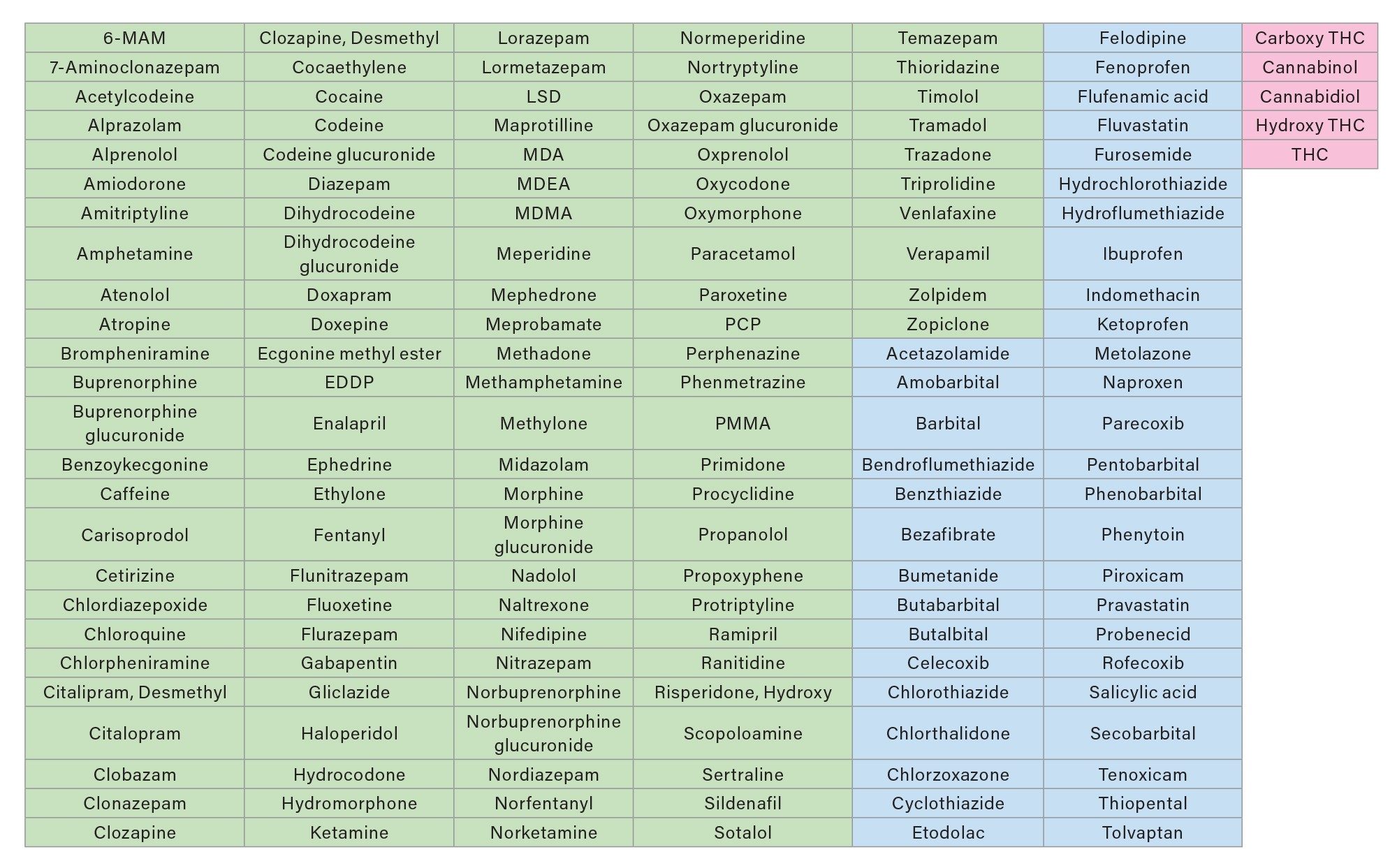
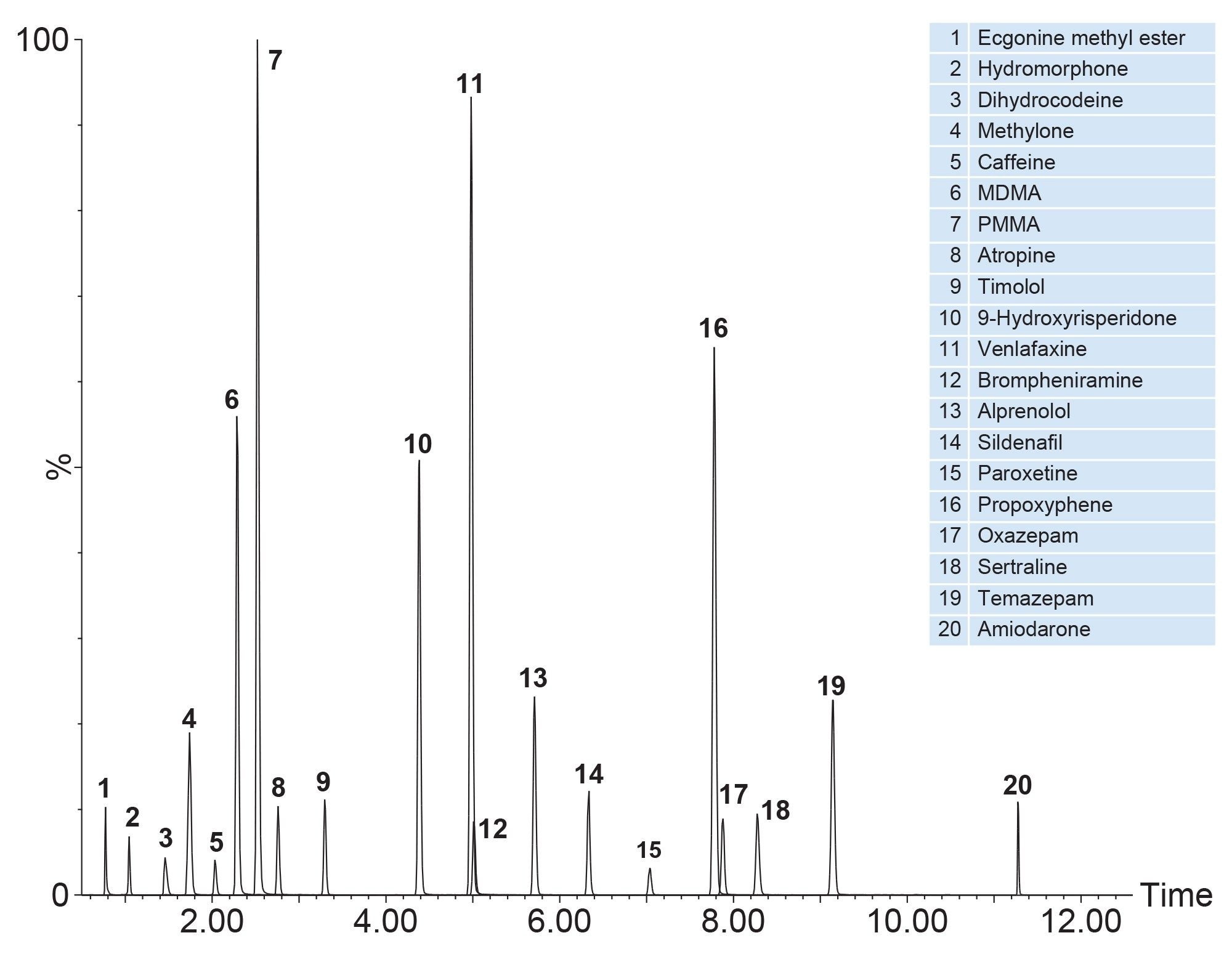
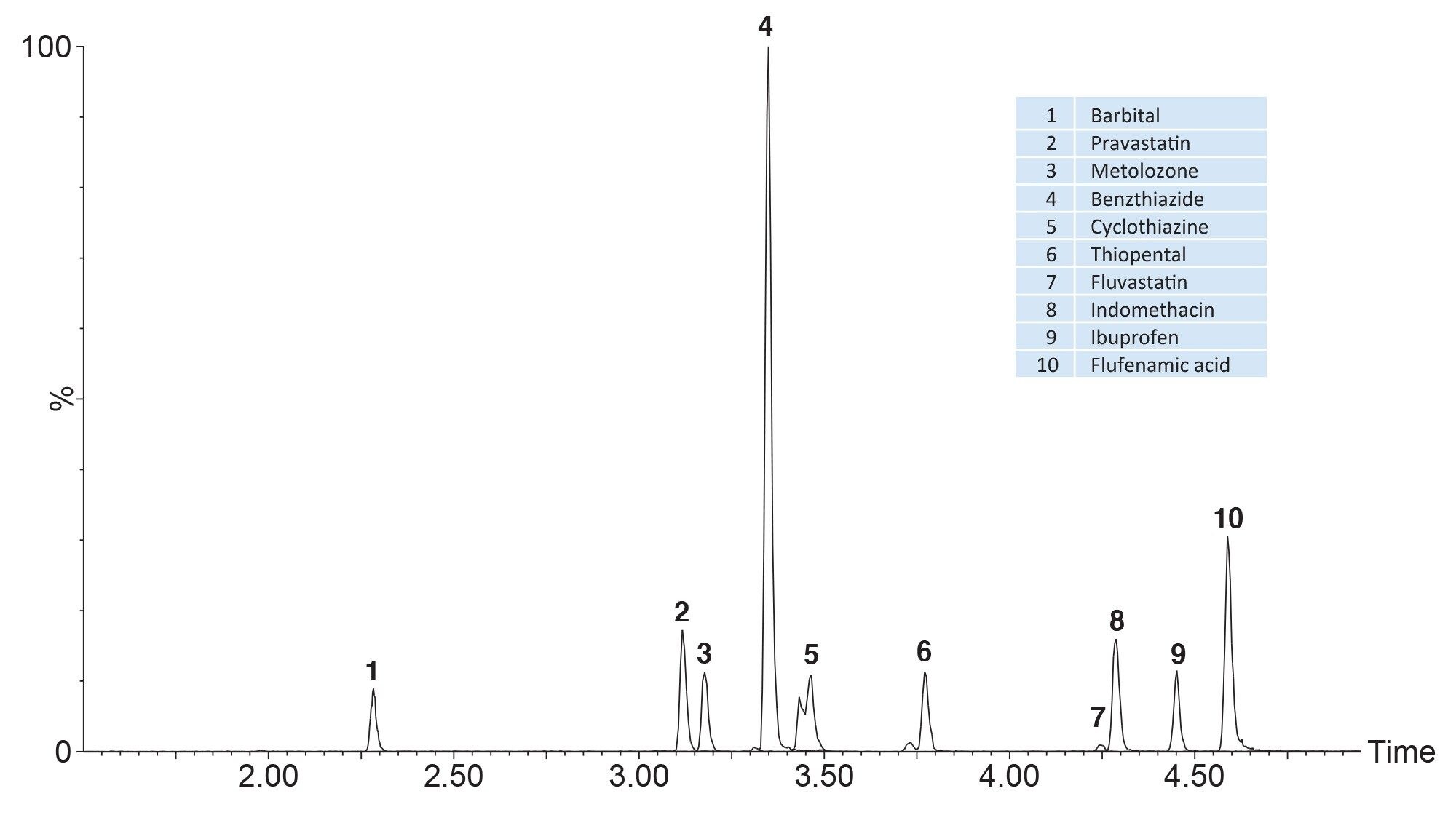
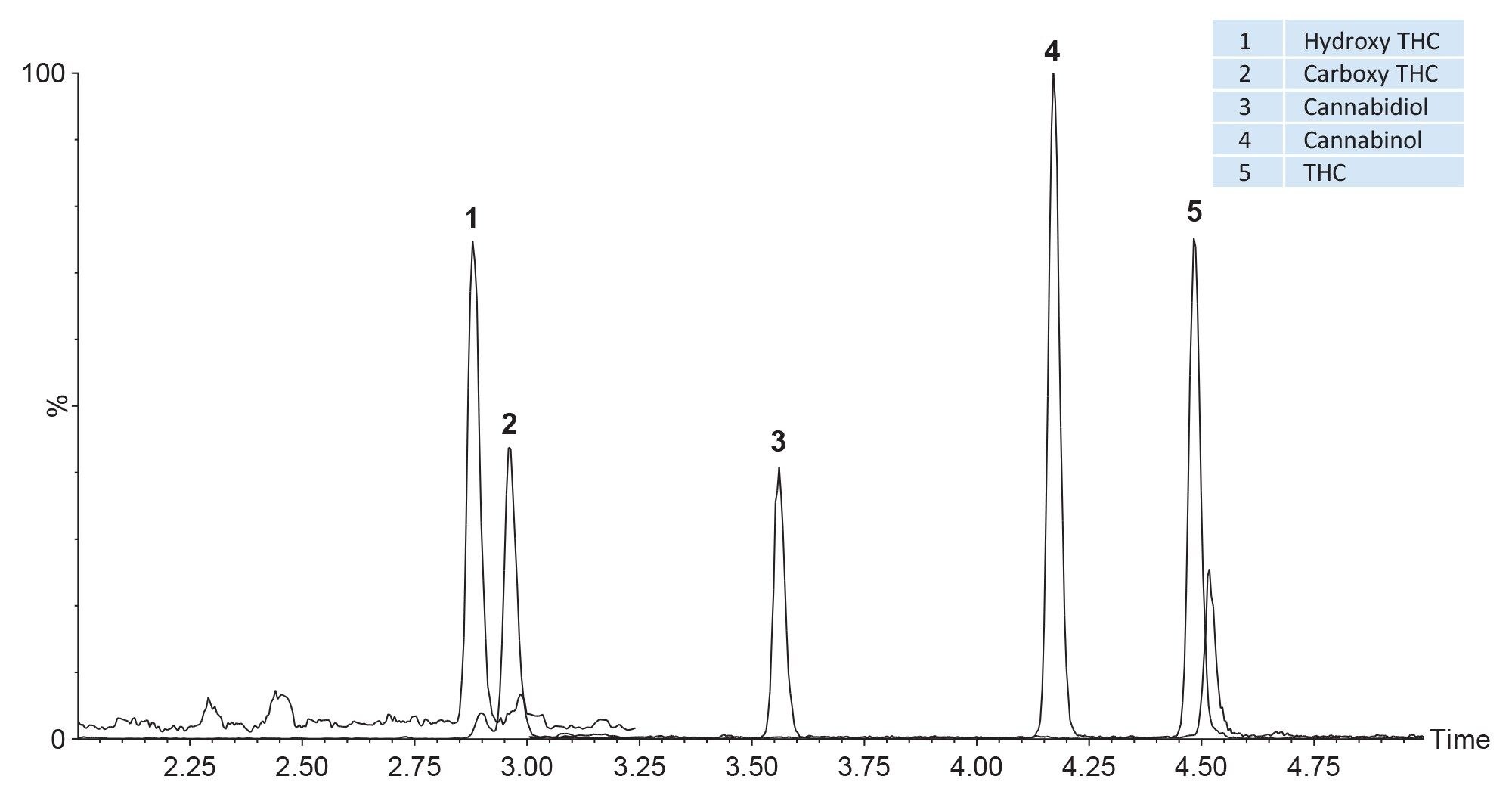
Figure 1 shows an example of a typical mixture containing twenty substances which were prepared at 10 ng/mL in whole blood, prepared using the pass-through sample preparation method as described and analysed using UPLC-MS/MS in ESI+. Figure 2 shows analysis of blood containing a mixture of negatively ionizing compounds spiked at 200 ng/mL and Figure 3 shows the resultant chromatogram for the cannabinoids spiked in whole blood at 10 ng/mL.
Conclusion
The increased use of whole blood for drug testing has highlighted the need for quick, accurate, reliable, and robust methods to screen for these compounds. This note details a simple, yet robust sample preparation procedure that has been successfully applied to a large panel of chemically diverse drug substances in whole blood. This generic sample preparation method may be useful for a broad range of testing scenarios.
The pass-through sample clean-up procedure is in microplate format and therefore also offers potential for automation if high sample throughput is required.
References
- Drug Driving (Specified Limits) (England and Wales) Regulations 2014 and the Drug Driving (Specified Limits) (England and Wales) (Amendment) Regulations 2015.
- Recommendations for Toxicological Investigation of Drug-Impaired Driving and Motor Vehicle Fatalities—2021 Update. A.L. D’Orazio et al., J. Anal. Toxicol., 45: 529-536 (2021).
- M. Wood and R. Lee. Analysis of Drugs in Blood to Support the Section 5A Driving Under the Influence of Drugs Act. Application Note, 720007451, 2021.
720007699, August 2022