
In this application note, the fast determination of residual glycerol and acylglycerols in model biodiesel prepared in-house using UltraPerformance Convergence Chromatography (UPC2) is discussed. This novel technology applies the performance advantages of UPLC such as speed, resolution, and sensitivity to supercritical fluid chromatography (SFC).
The development of new technologies that enable the production of fuels obtained from renewable resources is facilitated by both environmental concerns and a lack of fossil fuels. Biofuels, such as the biodiesel produced via trans-esterification of vegetable oils using either ethanol or methanol, are a promising alternative fuel source, particularly in countries with large territories and propitious weather for the desired agricultural activity, (e.g., Brazil). Compared to diesel, biodiesel can reduce 78% of CO2 emissions. Fatty acid alkyl esters (referred to here as commercial B100 biodiesel) that are used as automotive fuel for diesel engines should be free from lipid contaminants such as glycerol and acylglycerols.1 Residues in biodiesel that are less than 1% are anticipated to be triacylglycerols, diacylglycerols, monoacylglycerols, and free glycerol. The current technique for analysis of acylglycerols and glycerol involves either high-temperature GC or pre-derivatization, followed by conventional GC analysis. In this application note, the fast determination of residual glycerol and acylglycerols in model biodiesel prepared in-house using UltraPerformance Convergence Chromatography (UPC2) is discussed. This novel technology applies the performance advantages of UPLC such as speed, resolution, and sensitivity to supercritical fluid chromatography (SFC). Combining the use of supercritical CO2 with sub-2-μm particle columns, UPC2 represents an analysis technique that is orthogonal to reversed-phase LC and can be used to solve many troublesome separations that challenge conventional LC or GC analyses. With UPC2, no derivatization is required, resulting in easier and faster sample preparation and eliminating artifact formation. Unlike GC-MS, UPC2 allows for the analysis of compounds that can experience thermal degradation due to their low volatility.
Pure fatty acid ethyl esters (C16, C18, C18:1, C18:2, C18:3) were purchased from Sigma-Aldrich (St. Louis, MO) and mixed to form a model biodiesel. Pure C18 mono-acylglycerol, di-acylglycerol, and triacylglycerol plus glycerol and soybean oil were also obtained from Sigma Aldrich. All standards were prepared in 50:50 DCM/MeOH. Model biodiesel was prepared as a 5% (w/w) solution.
|
Column: |
ACQUITY UPC2 HSS C18 SB (3.0 x 150 mm, 1.8 μm) |
|
Samplep preparation: |
5% sample in DCM/MeOH |
|
ABPR: |
1500 psi |
|
Column temp.: |
25 °C |
|
Injection volume: |
2-8 μL |
|
Sample solvent: |
DCM/MeOH (50:50) |
|
Flow rate: |
1-2 mL/min |
|
Mobile phase A: |
Compressed CO2 |
|
Mobile phase B: |
Acetonitrile/methanol (90:10) |
|
Make up solvent: |
IPA |
|
Make up flow rate: |
0.2 mL/min |
|
Gradient: |
98:2 to 80:20 in 18 minutes |
|
Detectors: |
ACQUITY UPC2 PDA 210 nm, Ref. 400-500 nm ACQUITY UPC2 ELS Nebulizer: Cooling, Drift Tube: 50 °C, Gas Pressure: 40psi, Gain: 10, Make up flow is added to UPC2 column effluent, Split for BPR and ELSD is 1:3 |
A single ACQUITY UPC2 HSS C18 SB Column (3.0 x 150 mm) packed with 1.8-μm particles was used to separate glycerol, soybean oil acylglycerols, and model biodiesel components. A gradient of CO2 and acetonitrile/MeOH (90:10) served as the optimum mobile phase. First, the separation of model biodiesel, triacylglycerols, diacylglycerols, monoacylglycerols, and free glycerol was obtained (Figure 1). Baseline separation of all compounds was observed, which is important since most biodiesels have minor components that elute early and can interfere with proper quantitation of residual acylglycerols in the sample. Reproducibility of the separation was excellent for all analytes.
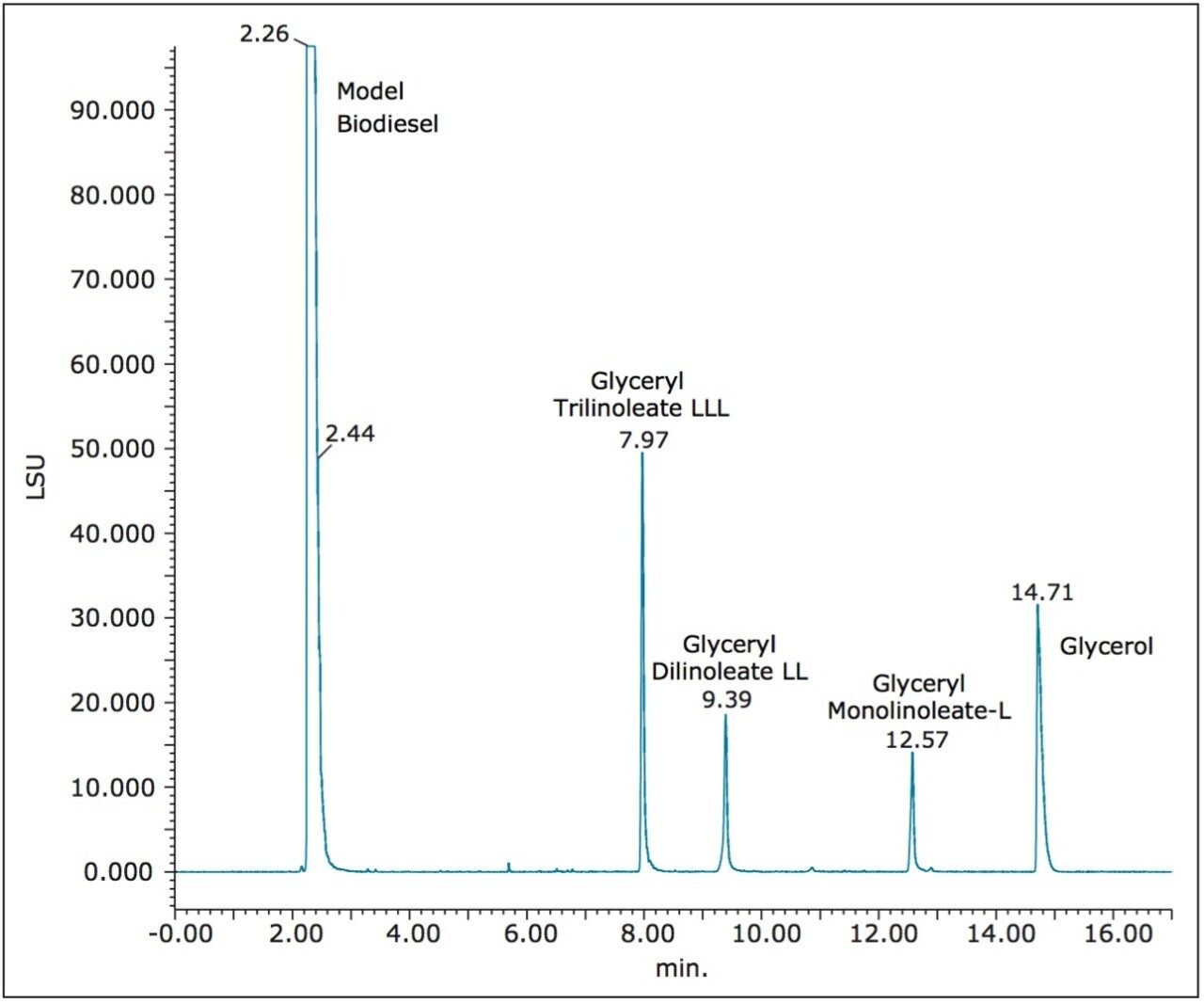
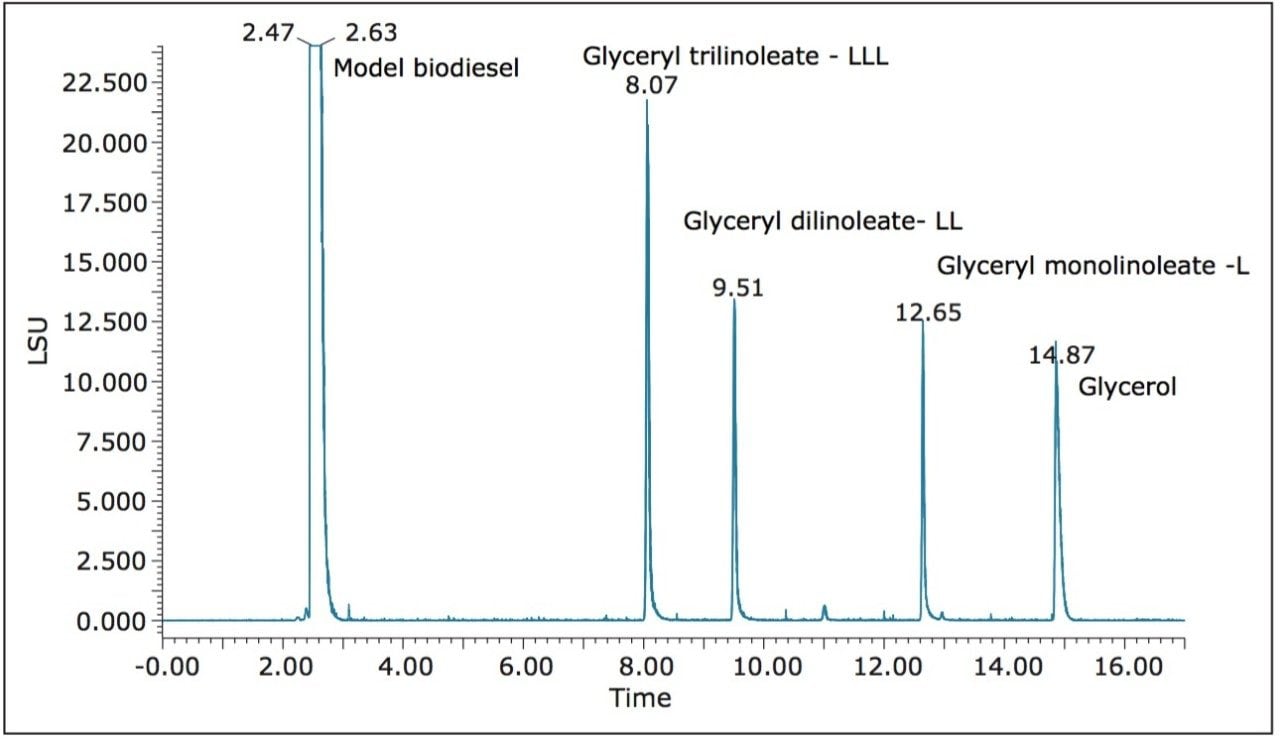
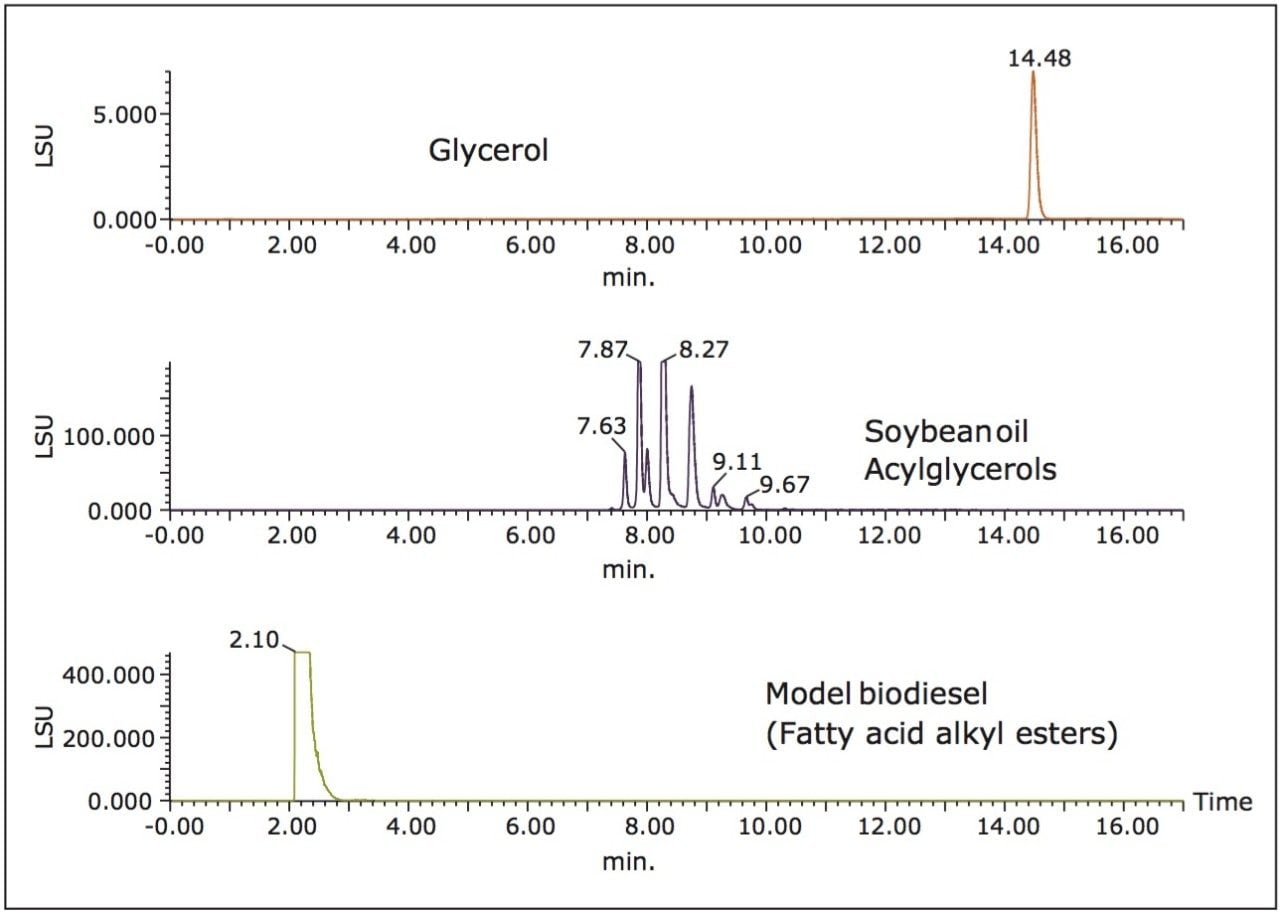
Figure 2 shows an overlay of six replicate injections of model biodiesel containing the four spiked impurities using UPC2 and ELS detection. RSD for all peak areas was between 1% and 4%, and RSD for retention time of all peaks was less than 0.1%. Next, chromatograms produced by single injection of the three classes of components are shown in Figure 3. Peaks from each class of compounds were resolved and no interferences were observed. A mixture prepared by spiking the model biodiesel with a known weight of soybean oil acylglycerols and glycerol was injected into the same column under the conditions noted above.
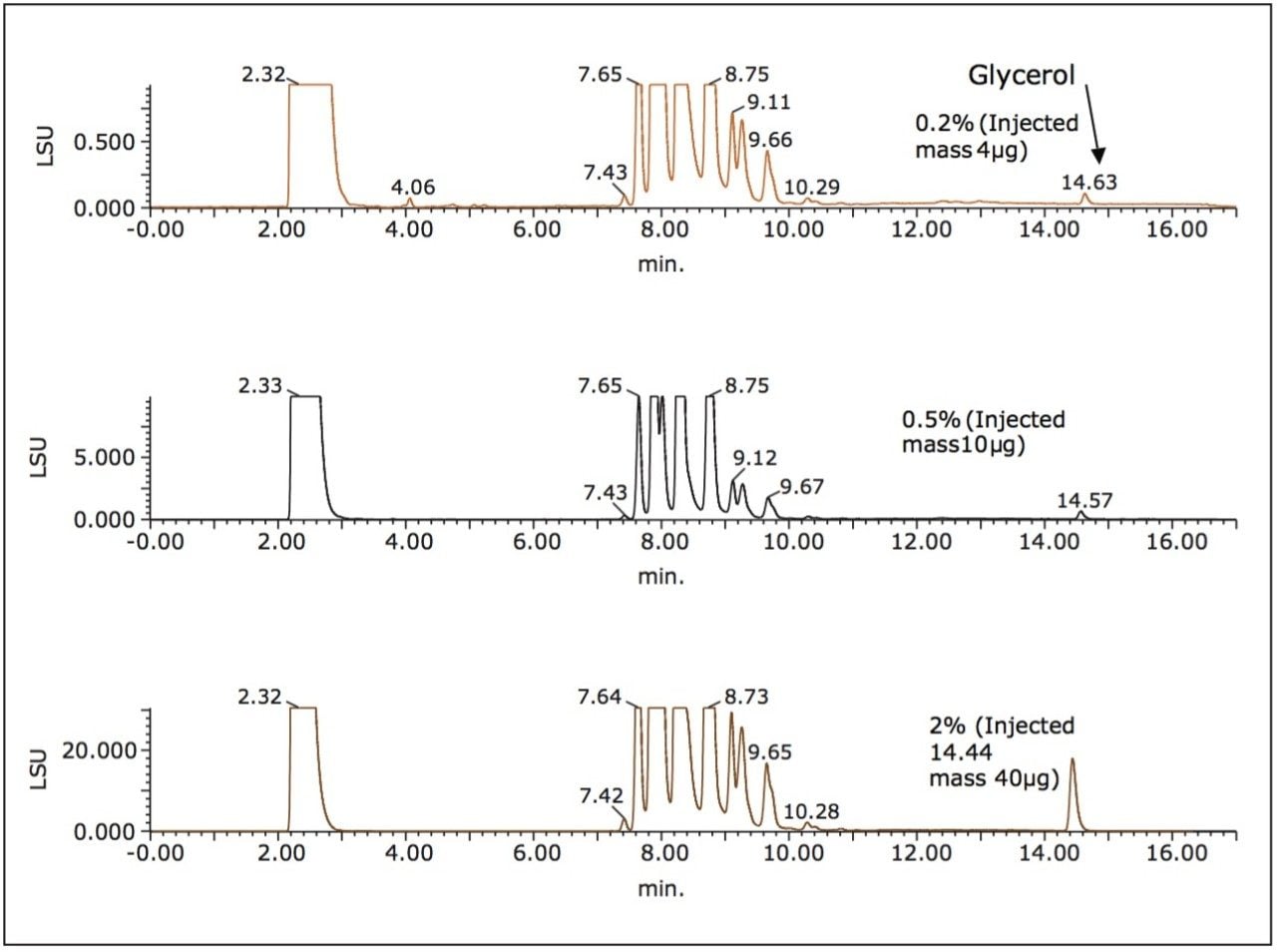
A series of chromatograms were generated wherein the spiking percentage was varied from 0.2% to 5.0% (Figure 4). At its lowest concentration (0.2%), the glycerol peak is clearly seen and well resolved from the other components for quantification purposes.
Larger injection volumes (4 and 8 μL) of the mixture spiked with impurities at 0.05% yielded comparable results ( Figure 5). The signal-to-noise (S/N) ratio for glycerol using ELS detection was about 10:1 at the 0.05% level with a 4-μL injection volume (2 μg mass injected on-column), and 40:1 at the same level with an 8-μL injection (4 μg injected on-column).

The ACQUITY UPC2 System with ELS detection was determined to be much faster and easier than either high-temperature GC or GC with preliminary derivatization as an analytical tool for determination of biodiesel impurities. Separation of triacylglycerols, diacylglycerols, monoacylglycerols, and free glycerol from model biodiesel at 5.0% to 0.2% was easily obtained. Glycerol was detected by ELS detection in model biodiesel with a S/N ratio of 10:1 at the 0.05% level with a 4-μL injection volume (2 μg mass on-column), and a S/N ratio of 40:1 for 4 μg injected on-column. A complete separation of all analytes was obtained in less than 15 minutes. UPC2 also gives the ability to analyze compounds with low volatility that would otherwise degrade at high temperature using GC-MS.
720004872, January 2014